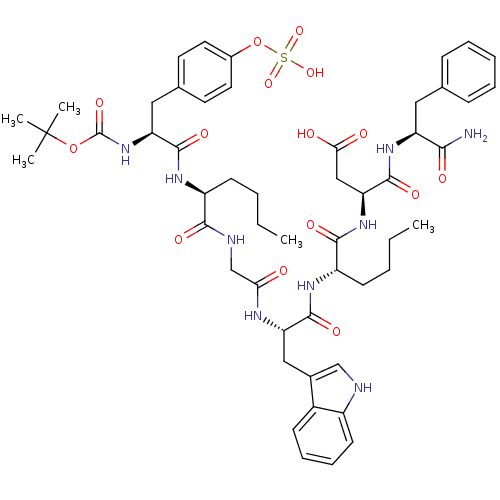
BDBM50016425 (S)-3-{(S)-2-[(S)-2-(2-{(S)-2-[(S)-2-tert-Butoxycarbonylamino-3-(4-sulfooxy-phenyl)-propionylamino]-hexanoylamino}-acetylamino)-3-(1H-indol-3-yl)-propionylamino]-hexanoylamino}-N-((S)-1-carbamoyl-2-phenyl-ethyl)-succinamic acid::3-{2-[2-(2-{2-[2-(tert-Butoxycarbonyl-methyl-amino)-3-(4-sulfonyl-oxy-phenyl)-propionylamino]-hexanoylamino}-acetylamino)-3-(1H-indol-3-yl)-propionylamino]-hexanoylamino}-N-(1-carbamoyl-2-phenyl-ethyl)-succinamic acid::3-{2-[2-(2-{2-[2-tert-Butoxycarbonylamino-3-(4-sulfooxy-phenyl)-propionylamino]-hexanoylamino}-acetylamino)-3-(1H-indol-3-yl)-propionylamino]-hexanoylamino}-N-(1-carbamoyl-2-phenyl-ethyl)-succinamic acid::Boc-Tyr(SO3H)-Nle-Gly-Trp-Nle-Asp-Phe-NH2::CHEMBL384035
SMILES CCCC[C@H](NC(=O)[C@H](Cc1ccc(OS(O)(=O)=O)cc1)NC(=O)OC(C)(C)C)C(=O)NCC(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)N[C@@H](CCCC)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](Cc1ccccc1)C(N)=O
InChI Key InChIKey=CSXIDZJPJYRGSK-UJKKYYSESA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 13 hits for monomerid = 50016425
Found 13 hits for monomerid = 50016425
TBA
Curated by ChEMBL
University Of Paris
Curated by ChEMBL
Centre De Pharmacologie-Endocrinologie (Montpellier, France)
Curated by ChEMBL
Centre De Pharmacologie-Endocrinologie (Montpellier, France)
Curated by ChEMBL
University Of Paris
Curated by ChEMBL
University Of Paris
Curated by ChEMBL
University Of Paris
Curated by ChEMBL