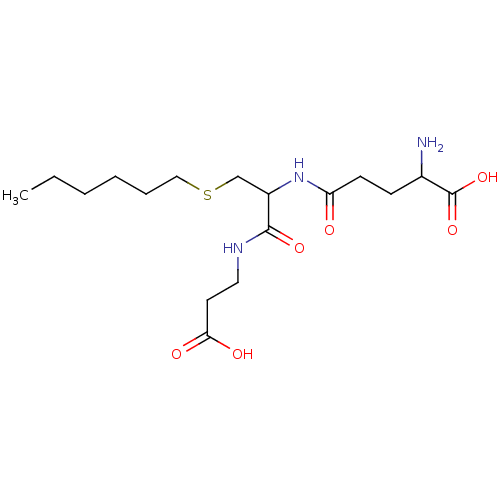
BDBM50043761 2-Amino-4-[1-(2-carboxy-ethylcarbamoyl)-2-hexylsulfanyl-ethylcarbamoyl]-butyric acid::CHEMBL60581
SMILES CCCCCCSCC(NC(=O)CCC(N)C(O)=O)C(=O)NCCC(O)=O
InChI Key InChIKey=RILVFYFKIDXJNY-UHFFFAOYSA-N
Data 4 KI
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 4 hits for monomerid = 50043761
Found 4 hits for monomerid = 50043761
Affinity DataKi: 1.10E+4nMAssay Description:Inhibitory activity of the compound was measured on recombinant human Glutathione S-transferase Mu 1More data for this Ligand-Target Pair
Affinity DataKi: 4.20E+4nMAssay Description:Inhibitory activity of the compound was measured on recombinant human Glutathione S-transferase Mu 2More data for this Ligand-Target Pair
Affinity DataKi: 4.30E+4nMAssay Description:Inhibitory activity of the compound was measured on recombinant human Glutathione-S-transferase A1 enzymeMore data for this Ligand-Target Pair
Affinity DataKi: 5.50E+5nMAssay Description:Inhibitory activity of the compound was measured on recombinant human Glutathione S-transferase PMore data for this Ligand-Target Pair