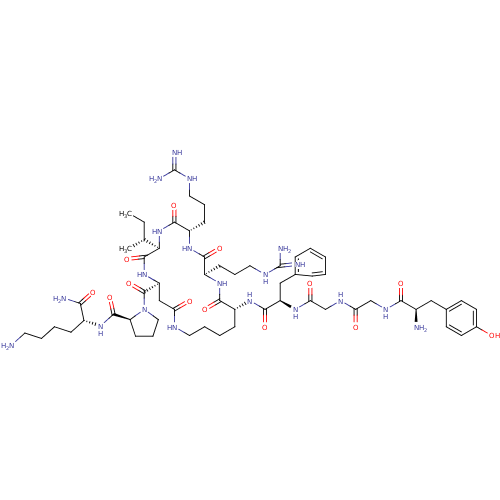
BDBM50051180 CHEMBL387083::Cyclic Lactam Peptide Analogues of Dynorphin A(1-11)-NH2
SMILES CC[C@@H](C)[C@H]1NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@@H](CCCNC(N)=N)NC(=O)[C@@H](CCCCNC(=O)C[C@H](NC1=O)C(=O)N1CCC[C@H]1C(=O)N[C@H](CCCCN)C(N)=O)NC(=O)[C@@H](Cc1ccccc1)NC(=O)CNC(=O)CNC(=O)[C@H](N)Cc1ccc(O)cc1
InChI Key InChIKey=AAMCVRWFZKNHTH-GILCVNLZSA-N
Data 3 IC50
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 3 hits for monomerid = 50051180
Found 3 hits for monomerid = 50051180
Affinity DataIC50: 50nMAssay Description:Inhibition of [3H]c[D-Pen2,p-Cl-Phe4,D-Pen5]enkephalin binding to delta opioid receptor of guinea pig brain plasma membrane homogenatesMore data for this Ligand-Target Pair
TargetKappa-type opioid receptor(Cavia porcellus (domestic guinea pig))
University Of Arizona
Curated by ChEMBL
University Of Arizona
Curated by ChEMBL
Affinity DataIC50: 6.60nMAssay Description:Inhibition of [3H]U-69,593 binding to Opioid receptor kappa 1 of plasma membrane homogenates of guinea pig brainMore data for this Ligand-Target Pair
Affinity DataIC50: 12nMAssay Description:Inhibition of [3H]-DAMGO binding to Opioid receptor mu 1 of guinea pig brain membrane homogenatesMore data for this Ligand-Target Pair