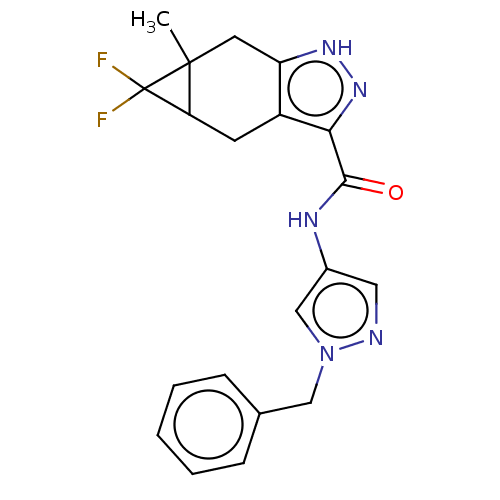
BDBM50086605 CHEMBL3426307
SMILES CC12Cc3[nH]nc(C(=O)Nc4cnn(Cc5ccccc5)c4)c3CC1C2(F)F
InChI Key
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 4 hits for monomerid = 50086605
Found 4 hits for monomerid = 50086605
Affinity DataKi: 0.300nMAssay Description:Inhibition of GST-tagged recombinant full length human ITK using AcEFPIYDFLPAKKK-NH2 as substrate after 35 mins by Morrison plot analysisMore data for this Ligand-Target Pair
Affinity DataKi: >400nMAssay Description:Inhibition of GST-tagged recombinant full length human ITK using AcEFPIYDFLPAKKK-NH2 as substrate after 35 mins by Morrison plot analysisMore data for this Ligand-Target Pair
Affinity DataKi: 600nMAssay Description:Inhibition of LCK (unknown origin)More data for this Ligand-Target Pair
Affinity DataIC50: 32nMAssay Description:Inhibition of ITK in TCR stimulated human Jurkat T cells assessed as reduction of PLC-gamma phosphorylation preincubated for 30 mins followed by TCR ...More data for this Ligand-Target Pair