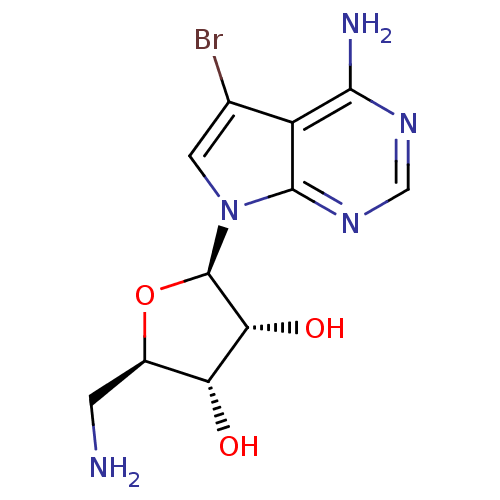
BDBM50090843 (1R,5S)-2-((3R,4aR)-4-Amino-5-bromo-pyrrolo[2,3-d]pyrimidin-7-yl)-5-aminomethyl-tetrahydro-furan-3,4-diol::(2R,3R,4S,5R)-2-(4-amino-5-bromo-7H-pyrrolo[2,3-d]pyrimidin-7-yl)-5-(aminomethyl)-tetrahydrofuran-3,4-diol::2-(4-Amino-5-bromo-pyrrolo[2,3-d]pyrimidin-7-yl)-5-aminomethyl-tetrahydro-furan-3,4-diol::CHEMBL99707
SMILES NC[C@H]1O[C@H]([C@H](O)[C@@H]1O)n1cc(Br)c2c(N)ncnc12
InChI Key InChIKey=SGHFHDYJYYPPIW-IOSLPCCCSA-N
Data 4 IC50
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 4 hits for monomerid = 50090843
Found 4 hits for monomerid = 50090843
Affinity DataIC50: 0.200nMAssay Description:Inhibition of recombinant human adenosine kinaseMore data for this Ligand-Target Pair
Affinity DataIC50: 2.00E+5nMAssay Description:Inhibition of adenosine kinase (unknown origin)More data for this Ligand-Target Pair
Affinity DataIC50: 0.200nMAssay Description:Concentration required for 50% inhibition of the adenosine kinase (AK) activity.More data for this Ligand-Target Pair
Affinity DataIC50: 0.200nMAssay Description:Inhibition of human adenosine kinase activityMore data for this Ligand-Target Pair