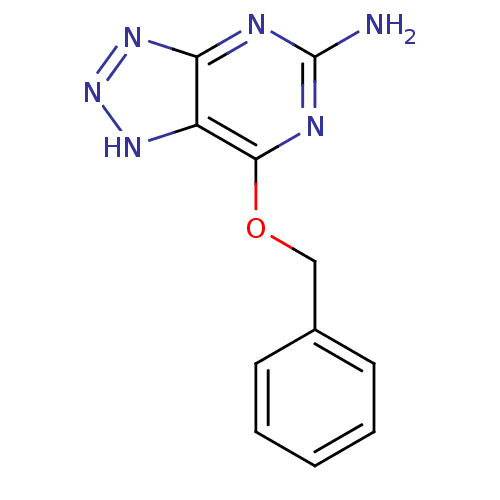
BDBM50106515 7-Benzyloxy-3H-[1,2,3]triazolo[4,5-d]pyrimidin-5-ylamine::CHEMBL333928
SMILES Nc1nc(OCc2ccccc2)c2[nH]nnc2n1
InChI Key InChIKey=ZHTBAYPUCDCQPX-UHFFFAOYSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 13 hits for monomerid = 50106515
Found 13 hits for monomerid = 50106515
TargetMethylated-DNA--protein-cysteine methyltransferase(Homo sapiens (Human))
German Cancer Research Center
Curated by ChEMBL
German Cancer Research Center
Curated by ChEMBL
Affinity DataIC50: 38nMAssay Description:In vitro inhibition of MGMT using cell free extracts from HeLa S3 cellsMore data for this Ligand-Target Pair
TargetMyeloperoxidase(Homo sapiens (Human))
Bristol-Myers Squibb Research And Development
Curated by ChEMBL
Bristol-Myers Squibb Research And Development
Curated by ChEMBL
Affinity DataIC50: 110nMAssay Description:Inhibition of human PMN leukocytes MPO peroxidation activity using H2O2 as substrate preincubated for 10 mins followed by H2O2 addition and measured ...More data for this Ligand-Target Pair
TargetLactoperoxidase(Homo sapiens (Human))
Bristol-Myers Squibb Research And Development
Curated by ChEMBL
Bristol-Myers Squibb Research And Development
Curated by ChEMBL
Affinity DataIC50: >1.00E+5nMAssay Description:Inhibition of human LPO assessed as reduction in H2O2 catalyzed 3,5-iodo tyrosine formation from 3-iodotyrosine and potassium iodide preincubated for...More data for this Ligand-Target Pair
Affinity DataIC50: 17nMAssay Description:Inhibition of human EPX bromination activity assessed as reduction in H2O2 catalyzed 3-bromo tyrosine formation from tyrosine and potassium bromide p...More data for this Ligand-Target Pair
TargetThyroid peroxidase(Homo sapiens (Human))
Bristol-Myers Squibb Research And Development
Curated by ChEMBL
Bristol-Myers Squibb Research And Development
Curated by ChEMBL
Affinity DataIC50: 4.20E+4nMAssay Description:Inhibition of human TPO assessed as reduction in H2O2 catalyzed 3,5-iodo tyrosine formation from 3-iodotyrosine and potassium iodide preincubated for...More data for this Ligand-Target Pair
TargetMyeloperoxidase(Homo sapiens (Human))
Bristol-Myers Squibb Research And Development
Curated by ChEMBL
Bristol-Myers Squibb Research And Development
Curated by ChEMBL
Affinity DataIC50: 110nMAssay Description:Inhibition of human PMN leukocytes MPO chlorination activity using H2O2 as substrate preincubated for 10 mins followed by H2O2 addition and measured ...More data for this Ligand-Target Pair
Affinity DataIC50: >1.00E+4nMAssay Description:Inhibition of CYP3A4 (unknown origin)More data for this Ligand-Target Pair
TargetMyeloperoxidase(Homo sapiens (Human))
Bristol-Myers Squibb Research And Development
Curated by ChEMBL
Bristol-Myers Squibb Research And Development
Curated by ChEMBL
Affinity DataIC50: 103nMAssay Description:Inhibition of recombinant human MPO incubated for 10 mins by amplex red dye based assayMore data for this Ligand-Target Pair
Affinity DataIC50: >1.00E+4nMAssay Description:Inhibition of CYP1A2 (unknown origin)More data for this Ligand-Target Pair
Affinity DataIC50: >1.00E+4nMAssay Description:Inhibition of CYP2B6 (unknown origin)More data for this Ligand-Target Pair
Affinity DataIC50: >1.00E+4nMAssay Description:Inhibition of CYP2C8 (unknown origin)More data for this Ligand-Target Pair
Affinity DataIC50: >1.00E+4nMAssay Description:Inhibition of CYP2D6 (unknown origin)More data for this Ligand-Target Pair
TargetMyeloperoxidase(Homo sapiens (Human))
Bristol-Myers Squibb Research And Development
Curated by ChEMBL
Bristol-Myers Squibb Research And Development
Curated by ChEMBL
Affinity DataIC50: 105nMAssay Description:Inhibition of recombinant human MPO incubated for 10 mins in presence of 240 mM NaCl and 10 uM H2O2 by aminophenyl fluorescein based assayMore data for this Ligand-Target Pair
 3D Structure (crystal)
3D Structure (crystal)