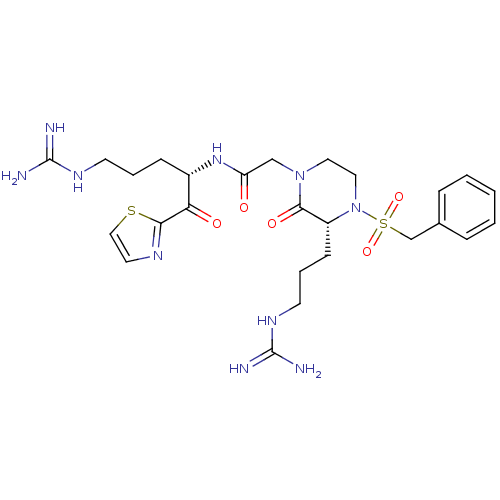
BDBM50125030 2-((R)-4-(benzylsulfonyl)-3-(3-guanidinopropyl)-2-oxopiperazin-1-yl)-N-((S)-5-guanidino-1-oxo-1-(thiazol-2-yl)pentan-2-yl)acetamide::2-[(R)-3-(3-Guanidino-propyl)-2-oxo-4-phenylmethanesulfonyl-piperazin-1-yl]-N-[(S)-4-guanidino-1-(thiazole-2-carbonyl)-butyl]-acetamide::CHEMBL350514
SMILES NC(=N)NCCC[C@H](NC(=O)CN1CCN([C@H](CCCNC(N)=N)C1=O)S(=O)(=O)Cc1ccccc1)C(=O)c1nccs1
InChI Key InChIKey=ABWTYHKQMAEDCC-VQTJNVASSA-N
Data 4 IC50
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 4 hits for monomerid = 50125030
Found 4 hits for monomerid = 50125030
Affinity DataIC50: 13nMAssay Description:In vitro inhibitory activity against factor XaMore data for this Ligand-Target Pair
Affinity DataIC50: 13nMAssay Description:Inhibition of human factor 10aMore data for this Ligand-Target Pair
TargetProthrombin(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Affinity DataIC50: 3.00E+3nMAssay Description:Inhibition of human alpha-thrombinMore data for this Ligand-Target Pair
TargetProthrombin(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Affinity DataIC50: 3.00E+3nMAssay Description:In vitro inhibitory activity against serine protease thrombinMore data for this Ligand-Target Pair