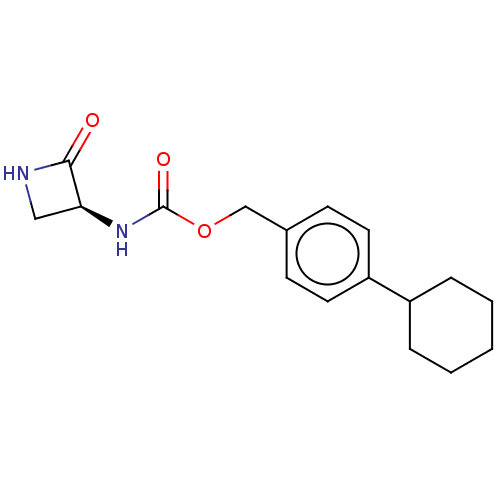
BDBM50151242 CHEMBL3770525
SMILES O=C(N[C@H]1CNC1=O)OCc1ccc(cc1)C1CCCCC1
InChI Key InChIKey=WSZKVBAJLRRRGX-HNNXBMFYSA-N
Data 3 IC50
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 3 hits for monomerid = 50151242
Found 3 hits for monomerid = 50151242
Affinity DataIC50: 1.51E+3nMAssay Description:Inhibition of human acid ceramidase by UPLC/MS analysisMore data for this Ligand-Target Pair
TargetN-acylethanolamine-hydrolyzing acid amidase(Homo sapiens (Human))
Italian Institute Of Technology
Curated by ChEMBL
Italian Institute Of Technology
Curated by ChEMBL
Affinity DataIC50: 12nMAssay Description:Inhibition of recombinant human NAAA expressed in HEK293 cells after 30 mins by UPLC/MS analysisMore data for this Ligand-Target Pair
TargetN-acylethanolamine-hydrolyzing acid amidase(Homo sapiens (Human))
Italian Institute Of Technology
Curated by ChEMBL
Italian Institute Of Technology
Curated by ChEMBL
Affinity DataIC50: 41nMAssay Description:Inhibition of human NAAA expressed in HEK293 cells preincubated for 10 mins followed by N-(4-methyl-2-oxo-chromen-7-yl)-hexadecanamide substrate addi...More data for this Ligand-Target Pair