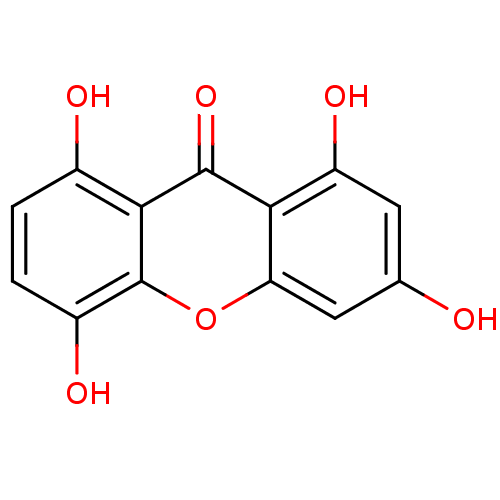
BDBM50155426 1,3,5,8-Tetrahydroxy-xanthen-9-one::1,3,5,8-tetrahydroxy-9H-xanthen-9-one::CHEMBL184574::DEMETHYLBELLIDIFOLIN::Xanthen-9-one, 1.0
SMILES Oc1cc(O)c2c(c1)oc1c(O)ccc(O)c1c2=O
InChI Key InChIKey=MPXAWSABMVLIBU-UHFFFAOYSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 4 hits for monomerid = 50155426
Found 4 hits for monomerid = 50155426
Affinity DataKi: 140nMAssay Description:Cdk5, 33P-ATP and cofactors were added in the presence of tau protein. The reaction mixture was incubated to allow Cdk5 to transfer 33P from ATP to ...More data for this Ligand-Target Pair
Affinity DataKi: 200nM IC50: 200nMAssay Description:Cdk5, 33P-ATP and cofactors were added in the presence of tau protein. The reaction mixture was incubated to allow Cdk5 to transfer 33P from ATP to ...More data for this Ligand-Target Pair
TargetCyclin-dependent kinase 5 activator 1(Homo sapiens (Human))
Harvard Medical School
Curated by ChEMBL
Harvard Medical School
Curated by ChEMBL
Affinity DataIC50: 200nMAssay Description:Inhibition of CDK5/P25 using full length tau as substrate by colorimetric ELISAMore data for this Ligand-Target Pair
Affinity DataIC50: 1.30E+4nMAssay Description:Concentration required to inhibit monoamine oxidase activity by 50%More data for this Ligand-Target Pair