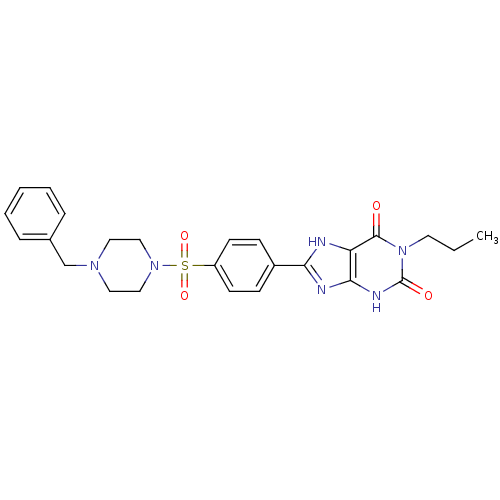
BDBM50190709 8-(4-(4-benzylpiperazin-1-ylsulfonyl)phenyl)-1-propyl-1H-purine-2,6(3H,7H)-dione::8-[4-(4-benzylpiperazide-1-sulfonyl)phenyl]-1-propylxanthine::CHEMBL212625
SMILES CCCn1c(=O)[nH]c2nc([nH]c2c1=O)-c1ccc(cc1)S(=O)(=O)N1CCN(Cc2ccccc2)CC1
InChI Key InChIKey=HHNGYTVWOXXKET-UHFFFAOYSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 23 hits for monomerid = 50190709
Found 23 hits for monomerid = 50190709
Affinity DataKi: 1.33nMAssay Description:Displacement of [3H]PSB-603 from human recombinant adenosine A2B receptor expressed in CHO cellsMore data for this Ligand-Target Pair
Affinity DataKi: 3.60nMAssay Description:Antagonist activity against human adenosine A2B receptorMore data for this Ligand-Target Pair
Affinity DataKi: 3.60nMAssay Description:Displacement of [3H]PSB298 from human recombinant adenosine A2B receptor expressed in CHO cellsMore data for this Ligand-Target Pair
Affinity DataKi: 3.60nMAssay Description:Binding affinity to human adenosine A2B receptorMore data for this Ligand-Target Pair
Affinity DataKi: 93.7nMAssay Description:Displacement of [3H]MSX2 from adenosine A2A receptor in rat brain striatal membranesMore data for this Ligand-Target Pair
Affinity DataKi: 93.7nMAssay Description:Binding affinity to rat adenosine A2A receptorMore data for this Ligand-Target Pair
Affinity DataKi: 93.7nMAssay Description:Displacement of [3H]MSX-2 from rat brain striatum adenosine A2A receptorMore data for this Ligand-Target Pair
Affinity DataKi: 260nMAssay Description:Displacement of [3H]CCPA from rat brain cortex adenosine A1 receptorMore data for this Ligand-Target Pair
Affinity DataKi: 260nMAssay Description:Displacement of [3H]CCPA from adenosine A1 receptor in rat brain cortical membranesMore data for this Ligand-Target Pair
Affinity DataKi: 260nMAssay Description:Binding affinity to rat adenosine A1 receptorMore data for this Ligand-Target Pair
Affinity DataKi: 484nMAssay Description:Displacement of [3H]MSX-2 from human recombinant adenosine A2A receptor expressed in CHO cellsMore data for this Ligand-Target Pair
Affinity DataKi: 484nMAssay Description:Displacement of [3H]MSX2 from human recombinant adenosine A2A receptor expressed in CHO cellsMore data for this Ligand-Target Pair
Affinity DataKi: 484nMAssay Description:Binding affinity to human adenosine A2A receptorMore data for this Ligand-Target Pair
Affinity DataKi: 484nMAssay Description:Antagonist activity against human adenosine A2A receptorMore data for this Ligand-Target Pair
Affinity DataKi: >1.00E+3nMAssay Description:Displacement of [3H]PSB-11 from human recombinant adenosine A3 receptor expressed in CHO cellsMore data for this Ligand-Target Pair
Affinity DataKi: >1.00E+3nMAssay Description:Displacement of [3H]PSB11 from human recombinant adenosine A3 receptor expressed in CHO cellsMore data for this Ligand-Target Pair
Affinity DataKi: >1.00E+3nMAssay Description:Antagonist activity against human adenosine A3 receptorMore data for this Ligand-Target Pair
Affinity DataKi: >1.00E+3nMAssay Description:Binding affinity to human adenosine A3 receptorMore data for this Ligand-Target Pair
Affinity DataKi: 2.07E+3nMAssay Description:Displacement of [3H]CCPA from human adenosine A1 receptor expressed in CHO cellsMore data for this Ligand-Target Pair
Affinity DataKi: 2.07E+3nMAssay Description:Displacement of [3H]CCPA from human recombinant adenosine A1 receptor expressed in CHO cellsMore data for this Ligand-Target Pair
Affinity DataKi: 2.07E+3nMAssay Description:Binding affinity to human adenosine A1 receptorMore data for this Ligand-Target Pair
Affinity DataKi: 2.07E+3nMAssay Description:Antagonist activity against human adenosine A1 receptorMore data for this Ligand-Target Pair
Affinity DataIC50: 10.1nMAssay Description:Antagonist activity at adenosine A2B receptor in human Jurkat T cells assessed as inhibition of NECA-induced increase in intracellular calcium concen...More data for this Ligand-Target Pair