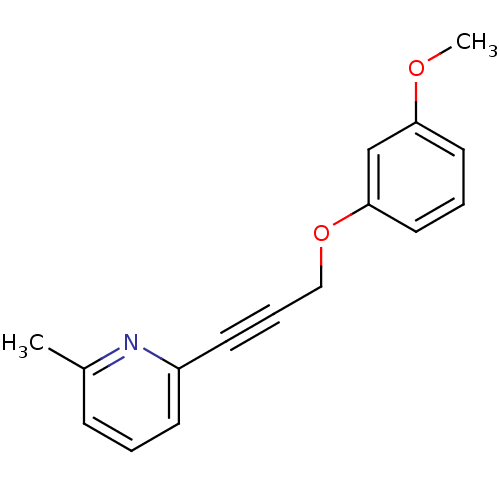
BDBM50191142 2-(3-(3-methoxyphenoxy)prop-1-ynyl)-6-methylpyridine::CHEMBL213102
SMILES COc1cccc(OCC#Cc2cccc(C)n2)c1
InChI Key InChIKey=AWOSYYCAGCWGCT-UHFFFAOYSA-N
Data 2 IC50
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 2 hits for monomerid = 50191142
Found 2 hits for monomerid = 50191142
TargetMetabotropic glutamate receptor 5(Homo sapiens (Human))
Astrazeneca R & D M£Lndal
Curated by ChEMBL
Astrazeneca R & D M£Lndal
Curated by ChEMBL
Affinity DataIC50: 397nMAssay Description:Activity at human mGluR5 assessed as effect on glutamate-induced calcium ion mobilization by FLIPRMore data for this Ligand-Target Pair
TargetMetabotropic glutamate receptor 1(Homo sapiens (Human))
Astrazeneca R & D M£Lndal
Curated by ChEMBL
Astrazeneca R & D M£Lndal
Curated by ChEMBL
Affinity DataIC50: >1.00E+4nMAssay Description:Activity at human mGluR1 assessed as effect on glutamate-induced calcium ion mobilization by FLIPRMore data for this Ligand-Target Pair