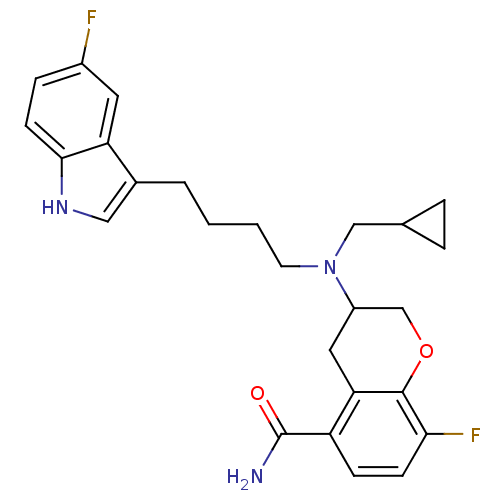
BDBM50191623 CHEMBL385353::rac-3-{(cyclopropylmethyl)[4-(5-fluoro-1H-indol-3-yl)butyl]amino}-8-fluorochromane-5-carboxamide
SMILES NC(=O)c1ccc(F)c2OCC(Cc12)N(CCCCc1c[nH]c2ccc(F)cc12)CC1CC1
InChI Key InChIKey=LLOLAUBGIFBYRO-UHFFFAOYSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 4 hits for monomerid = 50191623
Found 4 hits for monomerid = 50191623
Affinity DataKi: 2.10nMAssay Description:Displacement of [3H]-8-OH-DPAT from human 5HT1A receptor expressed in CHO cellsMore data for this Ligand-Target Pair
TargetSodium-dependent serotonin transporter(Rattus norvegicus (rat))
Wyeth Research
Curated by ChEMBL
Wyeth Research
Curated by ChEMBL
Affinity DataKi: 3.30nMAssay Description:Displacement of [3H]paroxetine from 5-HT transporter in Sprague-Dawley rat cortical membranesMore data for this Ligand-Target Pair
Affinity DataIC50: 51nMAssay Description:Agonist activity at human 5HT1A receptor expressed in CHO cells by inhibition of 8-OH-DPAT-induced decrease in forskolin-stimulated cAMP productionMore data for this Ligand-Target Pair
Affinity DataIC50: 82.4nMAssay Description:Inhibition of [3H]5-HT uptake at human 5-HT transporter expressed in Jar cellsMore data for this Ligand-Target Pair