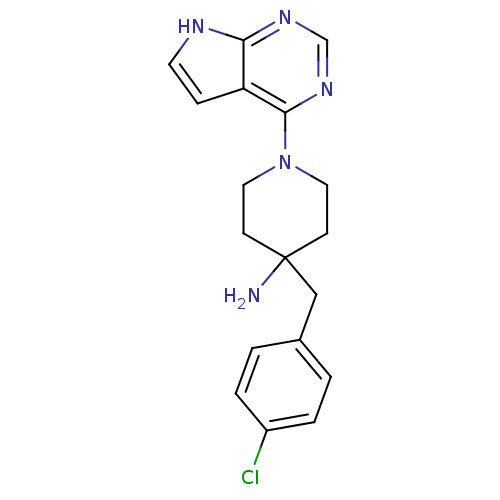
BDBM50237622 4-(4-Chlorobenzyl)-1-(7H-pyrrolo[2,3-d]pyrimidin-4-yl)piperidin-::4-(4-chlorobenzyl)-1-(7H-pyrrolo[2,3-d]pyrimidin-4-yl)piperidin-4-amine::CCT128930::CHEMBL263664::US8796293, 17
SMILES NC1(Cc2ccc(Cl)cc2)CCN(CC1)c1ncnc2[nH]ccc12
InChI Key InChIKey=RZIDZIGAXXNODG-UHFFFAOYSA-N
Data 21 IC50
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 21 hits for monomerid = 50237622
Found 21 hits for monomerid = 50237622
TargetRho-associated protein kinase 2(Homo sapiens (Human))
The Institute Of Cancer Research
US Patent
The Institute Of Cancer Research
US Patent
Affinity DataIC50: <1.00E+3nMpH: 7.5 T: 2°CAssay Description:In a final reaction volume of 25 ul, ROCK-II (h) (5-10 mU) is incubated with 50 mM Tris pH 7.5, 0.1 mM EGTA, 30 uM KEAKEKRQEQIAKRRRLSSLRASTSKSGGSQK (...More data for this Ligand-Target Pair
Affinity DataIC50: >5.00E+4nMAssay Description:Inhibition of CYP2E1More data for this Ligand-Target Pair
Affinity DataIC50: >5.00E+4nMAssay Description:Inhibition of CYP2A6More data for this Ligand-Target Pair
TargetGlycogen synthase kinase-3 beta(Homo sapiens (Human))
The Institute Of Cancer Research
Curated by ChEMBL
The Institute Of Cancer Research
Curated by ChEMBL
Affinity DataIC50: 3.00E+3nMAssay Description:Inhibition of GSK3-beta in human PC3M cells by ELISAMore data for this Ligand-Target Pair
Affinity DataIC50: 660nMAssay Description:Inhibition of CYP2D6 in human microsomal preparationMore data for this Ligand-Target Pair
TargetRAC-alpha serine/threonine-protein kinase(Homo sapiens (Human))
The Institute Of Cancer Research
Curated by ChEMBL
The Institute Of Cancer Research
Curated by ChEMBL
Affinity DataIC50: 0.660nMAssay Description:Inhibition of PKB in human U87MG cells assessed as GSK3beta phosphorylation by ELISAMore data for this Ligand-Target Pair
TargetRAC-alpha serine/threonine-protein kinase(Homo sapiens (Human))
The Institute Of Cancer Research
Curated by ChEMBL
The Institute Of Cancer Research
Curated by ChEMBL
Affinity DataIC50: 3nMAssay Description:Inhibition of PKB in human PC3M cells assessed as GSK3beta phosphorylation by ELISAMore data for this Ligand-Target Pair
TargetRAC-beta serine/threonine-protein kinase(Homo sapiens (Human))
The Institute Of Cancer Research
Curated by ChEMBL
The Institute Of Cancer Research
Curated by ChEMBL
Affinity DataIC50: 6nMAssay Description:Inhibition of PKBbeta by radiometric filter binding assayMore data for this Ligand-Target Pair
Affinity DataIC50: >1.00E+4nMAssay Description:Inhibition of CYP1A2 in human microsomal preparationMore data for this Ligand-Target Pair
Affinity DataIC50: >1.00E+4nMAssay Description:Inhibition of CYP3A4 in human microsomal preparationMore data for this Ligand-Target Pair
Affinity DataIC50: >1.00E+4nMAssay Description:Inhibition of CYP2C9 in human microsomal preparationMore data for this Ligand-Target Pair
Affinity DataIC50: >1.00E+4nMAssay Description:Inhibition of CYP2C19 in human microsomal preparationMore data for this Ligand-Target Pair
Affinity DataIC50: >5.00E+4nMAssay Description:Inhibition of CYP2C19More data for this Ligand-Target Pair
Affinity DataIC50: >5.00E+4nMAssay Description:Inhibition of CYP3A4More data for this Ligand-Target Pair
Affinity DataIC50: 1.00E+4nMAssay Description:Inhibition of CYP2C9More data for this Ligand-Target Pair
Affinity DataIC50: 1.00E+3nMAssay Description:Inhibition of CYP2D6More data for this Ligand-Target Pair
TargetRibosomal protein S6 kinase beta-1(Homo sapiens (Human))
The Institute Of Cancer Research
Curated by ChEMBL
The Institute Of Cancer Research
Curated by ChEMBL
Affinity DataIC50: 120nMAssay Description:Inhibition of p70S6K by radiometric filter binding assayMore data for this Ligand-Target Pair
TargetGlycogen synthase kinase-3 beta(Homo sapiens (Human))
The Institute Of Cancer Research
Curated by ChEMBL
The Institute Of Cancer Research
Curated by ChEMBL
Affinity DataIC50: 660nMAssay Description:Inhibition of GSK3-beta in human PC3M cells by ELISAMore data for this Ligand-Target Pair
TargetRAC-beta serine/threonine-protein kinase(Homo sapiens (Human))
The Institute Of Cancer Research
Curated by ChEMBL
The Institute Of Cancer Research
Curated by ChEMBL
Affinity DataIC50: 6nMAssay Description:Inhibition of PKBbeta recombinant by radiometric filter binding assayMore data for this Ligand-Target Pair
Affinity DataIC50: >5.00E+4nMAssay Description:Inhibition of CYP1A2More data for this Ligand-Target Pair
TargetRAC-alpha serine/threonine-protein kinase [E49C,C296S,C310S,C344S](Homo sapiens (Human))
Technische Universit??????T Dortmund
Technische Universit??????T Dortmund
Affinity DataIC50: 29nMAssay Description:IC50 determinations for activated Akt1 were measured with the KinEASE assay (Cisbio) according to the manufacturer’s instructions. The kinases A...More data for this Ligand-Target Pair