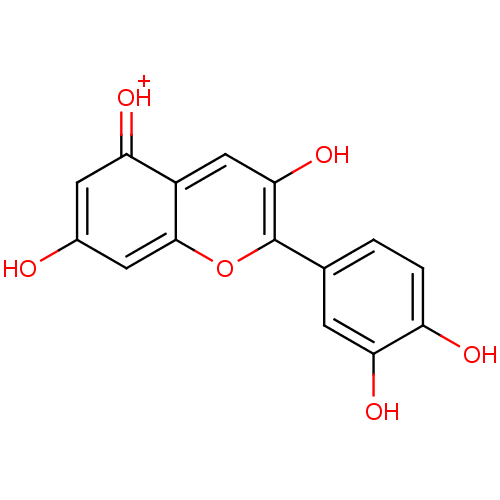
BDBM50241503 2-(3,4-Dihydroxyphenyl)-3,5,7-trihydroxy-1-Benzopyrylium::2-(3,4-dihydroxyphenyl)-3,5,7-trihydroxychromenylium::3,3',4',5,7-pentahydroxyflavylium::3,5,7,3'',4''-Pentahydroxyflavylium::CHEMBL404515::CHEMBL511367::Cyanidin::cyanidin(1+)
SMILES Oc1cc2oc(c(O)cc2c(=[OH+])c1)-c1ccc(O)c(O)c1
InChI Key InChIKey=QFCUROSHGOWZEG-UHFFFAOYSA-O
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 14 hits for monomerid = 50241503
Found 14 hits for monomerid = 50241503
Affinity DataEC50: 1.25E+5nMAssay Description:Agonist activity at human LXRbeta-LBD assessed as recruitment of co-activator peptide after 2 hrs by TR-FRET assayMore data for this Ligand-Target Pair
Affinity DataEC50: 3.50E+3nMAssay Description:Agonist activity at human LXRalpha-LBD assessed as recruitment of co-activator peptide after 2 hrs by TR-FRET assayMore data for this Ligand-Target Pair
Affinity DataKd: 7.32E+4nMAssay Description:Binding affinity to human LXRbeta-LBD by surface plasmon resonanceMore data for this Ligand-Target Pair
Affinity DataKd: 2.20E+3nMAssay Description:Binding affinity to human LXRalpha-LBD by surface plasmon resonanceMore data for this Ligand-Target Pair
Affinity DataKd: 7.80nMAssay Description:Inhibition of human thrombin assessed as equilibrium dissociation constant at 50 to 1000 uM by BIAcore analysisMore data for this Ligand-Target Pair
Affinity DataIC50: 250nMAssay Description:Inhibition of human thrombin amidolytic activity using D-Phe-Pip-Arg-pNA as substrate preincubated for 10 mins followed by substrate addition measure...More data for this Ligand-Target Pair
Affinity DataIC50: 4.00E+9nMAssay Description:Inhibition of baker's yeast alpha-glucosidase using p-nitrophenyl-alpha-D-glucopyranoside as substrate by spectrophotometryMore data for this Ligand-Target Pair
TargetProstaglandin G/H synthase 1(Homo sapiens (Human))
Department Of Horticulture And National Food Safety And Toxicology Center
Curated by ChEMBL
Department Of Horticulture And National Food Safety And Toxicology Center
Curated by ChEMBL
Affinity DataIC50: 9.00E+7nMAssay Description:Inhibition of PGHS1 assessed as conversion of arachidonic acid to prostaglandinMore data for this Ligand-Target Pair
Affinity DataIC50: 1.50E+5nMAssay Description:Displacement of ANS from DAPK1 catalytic domain (1 to 285) (unknown origin) after 30 mins by fluorescence assayMore data for this Ligand-Target Pair
Affinity DataIC50: 1.17E+4nMAssay Description:Inhibition of human recombinant glyoxalase 1 assessed as S-D-lactoylglutathione after 15 mins by spectrophotometric analysisMore data for this Ligand-Target Pair
TargetADP-ribosyl cyclase/cyclic ADP-ribose hydrolase 1(Homo sapiens (Human))
University Of Strasburg
Curated by ChEMBL
University Of Strasburg
Curated by ChEMBL
Affinity DataIC50: 2.18E+4nMAssay Description:Inhibition of human CD38 using 20 uM 1, N6-etheno NAD+ as substrate by continuous fluorimetric methodMore data for this Ligand-Target Pair
Affinity DataIC50: 6.00E+7nMAssay Description:Inhibition of COX2More data for this Ligand-Target Pair
TargetProstaglandin G/H synthase 1(Homo sapiens (Human))
Department Of Horticulture And National Food Safety And Toxicology Center
Curated by ChEMBL
Department Of Horticulture And National Food Safety And Toxicology Center
Curated by ChEMBL
Affinity DataIC50: 9.00E+7nMAssay Description:Inhibition of COX1More data for this Ligand-Target Pair
Affinity DataIC50: 6.00E+7nMAssay Description:Inhibition of PGHS2 assessed as conversion of arachidonic acid to prostaglandinMore data for this Ligand-Target Pair