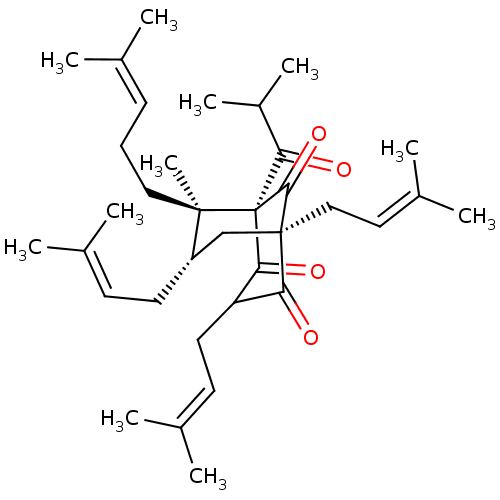
BDBM50242259 CHEMBL516641::HYPERFORIN
SMILES [#6]-[#6](-[#6])-[#6](=O)[C@]12[#6](=O)-[#6](-[#6]\[#6]=[#6](/[#6])-[#6])-[#6](=O)[C@@]([#6]\[#6]=[#6](\[#6])-[#6])([#6]-[#6@H](-[#6]\[#6]=[#6](/[#6])-[#6])[C@@]1([#6])[#6]-[#6]\[#6]=[#6](/[#6])-[#6])[#6]2=O
InChI Key InChIKey=GQRREYKSPJMLAW-CWRBZUMDSA-N
Data 38 KI
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 38 hits for monomerid = 50242259
Found 38 hits for monomerid = 50242259
TargetD(1A) dopamine receptor(Homo sapiens (Human))
WestfÄLische Wilhelms-UniversitÄ
Curated by PDSP Ki Database
WestfÄLische Wilhelms-UniversitÄ
Curated by PDSP Ki Database
TargetD(1B) dopamine receptor(Homo sapiens (Human))
WestfÄLische Wilhelms-UniversitÄ
Curated by PDSP Ki Database
WestfÄLische Wilhelms-UniversitÄ
Curated by PDSP Ki Database
TargetGamma-aminobutyric acid receptor subunit alpha-1(Rattus norvegicus (Rat))
WestfÄLische Wilhelms-UniversitÄ
Curated by PDSP Ki Database
WestfÄLische Wilhelms-UniversitÄ
Curated by PDSP Ki Database
TargetGlutamate receptor ionotropic, NMDA 1(RAT)
WestfÄLische Wilhelms-UniversitÄ
Curated by PDSP Ki Database
WestfÄLische Wilhelms-UniversitÄ
Curated by PDSP Ki Database
TargetGlutamate receptor ionotropic, NMDA 1(RAT)
WestfÄLische Wilhelms-UniversitÄ
Curated by PDSP Ki Database
WestfÄLische Wilhelms-UniversitÄ
Curated by PDSP Ki Database
TargetSodium-dependent noradrenaline transporter(Homo sapiens (Human))
WestfÄLische Wilhelms-UniversitÄ
Curated by PDSP Ki Database
WestfÄLische Wilhelms-UniversitÄ
Curated by PDSP Ki Database
TargetDelta-type opioid receptor(GUINEA PIG)
WestfÄLische Wilhelms-UniversitÄ
Curated by PDSP Ki Database
WestfÄLische Wilhelms-UniversitÄ
Curated by PDSP Ki Database
TargetKappa-type opioid receptor(Homo sapiens (Human))
WestfÄLische Wilhelms-UniversitÄ
Curated by PDSP Ki Database
WestfÄLische Wilhelms-UniversitÄ
Curated by PDSP Ki Database
TargetMu-type opioid receptor(Homo sapiens (Human))
WestfÄLische Wilhelms-UniversitÄ
Curated by PDSP Ki Database
WestfÄLische Wilhelms-UniversitÄ
Curated by PDSP Ki Database
TargetVasopressin V1b receptor(Homo sapiens (Human))
WestfÄLische Wilhelms-UniversitÄ
Curated by PDSP Ki Database
WestfÄLische Wilhelms-UniversitÄ
Curated by PDSP Ki Database
TargetVasopressin V2 receptor(Homo sapiens (Human))
WestfÄLische Wilhelms-UniversitÄ
Curated by PDSP Ki Database
WestfÄLische Wilhelms-UniversitÄ
Curated by PDSP Ki Database
TargetVasopressin V1b receptor(Homo sapiens (Human))
WestfÄLische Wilhelms-UniversitÄ
Curated by PDSP Ki Database
WestfÄLische Wilhelms-UniversitÄ
Curated by PDSP Ki Database
TargetSodium-dependent serotonin transporter(Homo sapiens (Human))
WestfÄLische Wilhelms-UniversitÄ
Curated by PDSP Ki Database
WestfÄLische Wilhelms-UniversitÄ
Curated by PDSP Ki Database
Target5-hydroxytryptamine receptor 1A(Homo sapiens (Human))
WestfÄLische Wilhelms-UniversitÄ
Curated by PDSP Ki Database
WestfÄLische Wilhelms-UniversitÄ
Curated by PDSP Ki Database
Target5-hydroxytryptamine receptor 1D(Homo sapiens (Human))
WestfÄLische Wilhelms-UniversitÄ
Curated by PDSP Ki Database
WestfÄLische Wilhelms-UniversitÄ
Curated by PDSP Ki Database
Target5-hydroxytryptamine receptor 2C(Rattus norvegicus (Rat))
WestfÄLische Wilhelms-UniversitÄ
Curated by PDSP Ki Database
WestfÄLische Wilhelms-UniversitÄ
Curated by PDSP Ki Database
Target5-hydroxytryptamine receptor 3A(Homo sapiens (Human))
WestfÄLische Wilhelms-UniversitÄ
Curated by PDSP Ki Database
WestfÄLische Wilhelms-UniversitÄ
Curated by PDSP Ki Database
Target5-hydroxytryptamine receptor 5A(Homo sapiens (Human))
WestfÄLische Wilhelms-UniversitÄ
Curated by PDSP Ki Database
WestfÄLische Wilhelms-UniversitÄ
Curated by PDSP Ki Database
Target5-hydroxytryptamine receptor 6(Homo sapiens (Human))
WestfÄLische Wilhelms-UniversitÄ
Curated by PDSP Ki Database
WestfÄLische Wilhelms-UniversitÄ
Curated by PDSP Ki Database
Target5-hydroxytryptamine receptor 7(Homo sapiens (Human))
WestfÄLische Wilhelms-UniversitÄ
Curated by PDSP Ki Database
WestfÄLische Wilhelms-UniversitÄ
Curated by PDSP Ki Database
TargetAlpha-1A adrenergic receptor(Homo sapiens (Human))
WestfÄLische Wilhelms-UniversitÄ
Curated by PDSP Ki Database
WestfÄLische Wilhelms-UniversitÄ
Curated by PDSP Ki Database
TargetAlpha-1B adrenergic receptor(Homo sapiens (Human))
WestfÄLische Wilhelms-UniversitÄ
Curated by PDSP Ki Database
WestfÄLische Wilhelms-UniversitÄ
Curated by PDSP Ki Database
TargetAlpha-2A adrenergic receptor(Homo sapiens (Human))
WestfÄLische Wilhelms-UniversitÄ
Curated by PDSP Ki Database
WestfÄLische Wilhelms-UniversitÄ
Curated by PDSP Ki Database
TargetAlpha-2B adrenergic receptor(Homo sapiens (Human))
WestfÄLische Wilhelms-UniversitÄ
Curated by PDSP Ki Database
WestfÄLische Wilhelms-UniversitÄ
Curated by PDSP Ki Database
TargetAlpha-2C adrenergic receptor(Homo sapiens (Human))
WestfÄLische Wilhelms-UniversitÄ
Curated by PDSP Ki Database
WestfÄLische Wilhelms-UniversitÄ
Curated by PDSP Ki Database
TargetBeta-1 adrenergic receptor(Rattus norvegicus (Rat))
WestfÄLische Wilhelms-UniversitÄ
Curated by PDSP Ki Database
WestfÄLische Wilhelms-UniversitÄ
Curated by PDSP Ki Database
TargetBeta-2 adrenergic receptor(Rattus norvegicus)
WestfÄLische Wilhelms-UniversitÄ
Curated by PDSP Ki Database
WestfÄLische Wilhelms-UniversitÄ
Curated by PDSP Ki Database
TargetMuscarinic acetylcholine receptor M1(Homo sapiens (Human))
WestfÄLische Wilhelms-UniversitÄ
Curated by PDSP Ki Database
WestfÄLische Wilhelms-UniversitÄ
Curated by PDSP Ki Database
TargetMuscarinic acetylcholine receptor M2(Homo sapiens (Human))
WestfÄLische Wilhelms-UniversitÄ
Curated by PDSP Ki Database
WestfÄLische Wilhelms-UniversitÄ
Curated by PDSP Ki Database
TargetMuscarinic acetylcholine receptor M3(Homo sapiens (Human))
WestfÄLische Wilhelms-UniversitÄ
Curated by PDSP Ki Database
WestfÄLische Wilhelms-UniversitÄ
Curated by PDSP Ki Database
TargetMuscarinic acetylcholine receptor M4(Homo sapiens (Human))
WestfÄLische Wilhelms-UniversitÄ
Curated by PDSP Ki Database
WestfÄLische Wilhelms-UniversitÄ
Curated by PDSP Ki Database
TargetMuscarinic acetylcholine receptor M5(Homo sapiens (Human))
WestfÄLische Wilhelms-UniversitÄ
Curated by PDSP Ki Database
WestfÄLische Wilhelms-UniversitÄ
Curated by PDSP Ki Database
TargetD(2) dopamine receptor(Rattus norvegicus (rat))
WestfÄLische Wilhelms-UniversitÄ
Curated by PDSP Ki Database
WestfÄLische Wilhelms-UniversitÄ
Curated by PDSP Ki Database
TargetD(3) dopamine receptor(Rattus norvegicus (Rat))
WestfÄLische Wilhelms-UniversitÄ
Curated by PDSP Ki Database
WestfÄLische Wilhelms-UniversitÄ
Curated by PDSP Ki Database
TargetSodium-dependent dopamine transporter(BOVINE)
WestfÄLische Wilhelms-UniversitÄ
Curated by PDSP Ki Database
WestfÄLische Wilhelms-UniversitÄ
Curated by PDSP Ki Database
TargetGamma-aminobutyric acid receptor subunit alpha-1(Rattus norvegicus (Rat))
WestfÄLische Wilhelms-UniversitÄ
Curated by PDSP Ki Database
WestfÄLische Wilhelms-UniversitÄ
Curated by PDSP Ki Database