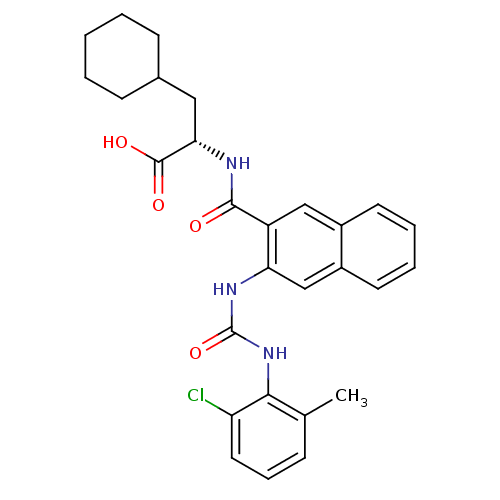
BDBM50243305 (S)-2-(2-(3-(2-chloro-6-methylphenyl)ureido)-2-naphthamido)-3-cyclohexylpropanoic acid::CHEMBL507762
SMILES Cc1cccc(Cl)c1NC(=O)Nc1cc2ccccc2cc1C(=O)N[C@@H](CC1CCCCC1)C(O)=O
InChI Key InChIKey=UEAKFJHZEFJNCU-DEOSSOPVSA-N
Data 4 IC50
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 4 hits for monomerid = 50243305
Found 4 hits for monomerid = 50243305
Affinity DataIC50: 2.20E+3nMAssay Description:Inhibition of human glycogen phosphorylase alpha in presence of glucoseMore data for this Ligand-Target Pair
Affinity DataIC50: 7.40E+3nMAssay Description:Inhibition of human glycogen phosphorylase alpha in HepG2 cells assessed as inhibition of forskolin-induced glycogenolysis after 60 minsMore data for this Ligand-Target Pair
Affinity DataIC50: 2.20E+3nMAssay Description:Inhibition of human glycogen phosphorylase alphaMore data for this Ligand-Target Pair
Affinity DataIC50: 5.10E+4nMAssay Description:Inhibition of human glycogen phosphorylase alpha in absence of glucoseMore data for this Ligand-Target Pair