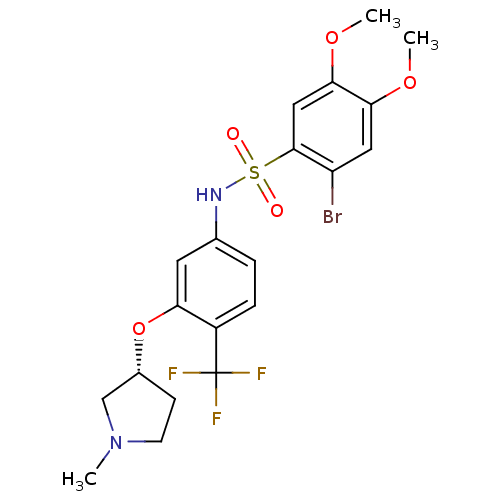
BDBM50249878 (R)-2-bromo-4,5-dimethoxy-N-(3-(1-methylpyrrolidin-3-yloxy)-4-(trifluoromethyl)phenyl)benzenesulfonamide::CHEMBL522770::SB-706375
SMILES COc1cc(Br)c(cc1OC)S(=O)(=O)Nc1ccc(c(O[C@@H]2CCN(C)C2)c1)C(F)(F)F
InChI Key InChIKey=BPOWQJYAMDEAFF-CYBMUJFWSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 6 hits for monomerid = 50249878
Found 6 hits for monomerid = 50249878
Affinity DataKi: 5.40nMAssay Description:Binding affinity to urotensin2 receptor (unknown origin)More data for this Ligand-Target Pair
TargetUrotensin-2 receptor(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Affinity DataKi: 8nMAssay Description:Displacement of [125I]urotensin 2 from urotensin 2 receptor in human RMS13 cells by scintillation proximity assayMore data for this Ligand-Target Pair
TargetUrotensin-2 receptor(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Affinity DataIC50: 14nMAssay Description:Antagonist activity at recombinant human urotensin2 receptor expressed in CHO cells assessed as inhibition of urotensin2-stimulated Ca2+ mobilization...More data for this Ligand-Target Pair
TargetUrotensin-2 receptor(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Affinity DataIC50: 8nMAssay Description:Antagonist activity at urotensin 2 receptor in human RMS13 cells assessed as inhibition of urotensin 2-induced intracellular calcium mobilization aft...More data for this Ligand-Target Pair
TargetUrotensin-2 receptor(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Affinity DataIC50: 6nMAssay Description:Displacement of [125I]-U2 from human recombinant urotensin2 receptor expressed in human Chem-2 cells after 4 hrs by scintillation proximity assayMore data for this Ligand-Target Pair
TargetUrotensin-2 receptor(RAT)
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Affinity DataIC50: 34nMAssay Description:Antagonist activity at rat urotensin 2 receptor expressed in CHOK1 cells assessed as inhibition of urotensin 2-induced intracellular calcium mobiliza...More data for this Ligand-Target Pair