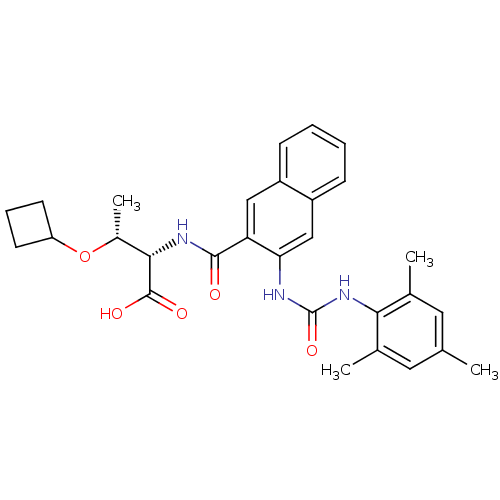
BDBM50256668 (2S,3R)-3-cyclobutoxy-2-(3-(3-mesitylureido)-2-naphthamido)butanoic acid::CHEMBL473852
SMILES C[C@@H](OC1CCC1)[C@H](NC(=O)c1cc2ccccc2cc1NC(=O)Nc1c(C)cc(C)cc1C)C(O)=O
InChI Key InChIKey=VFXCDESXOOWVLU-BCHFMIIMSA-N
Data 3 IC50
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 3 hits for monomerid = 50256668
Found 3 hits for monomerid = 50256668
Affinity DataIC50: 434nMAssay Description:Inhibition of liver glycogen phosphorylase A in human HepG2 cells assessed as inhibition of forskolin-induced glucogenolysisMore data for this Ligand-Target Pair
Affinity DataIC50: 17nMAssay Description:Inhibition of human liver glycogen phosphorylase A by fluorescence plate reader assayMore data for this Ligand-Target Pair
Affinity DataIC50: 2.10E+3nMAssay Description:Inhibition of CYP2C9 (unknown origin)More data for this Ligand-Target Pair