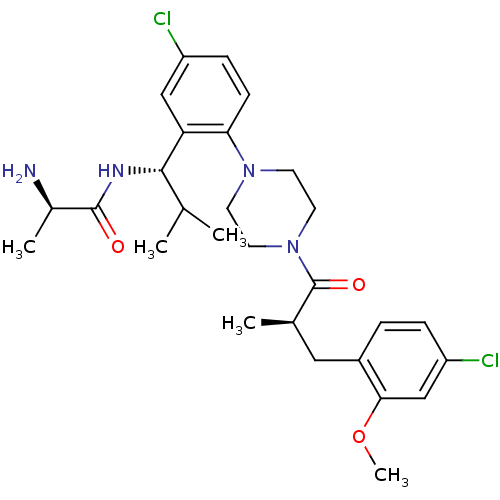
BDBM50261229 1-{2-[(1S)-((2R)-Aminopropionamido)-2-methylpropyl]-4-chlorophenyl}-4-[(2R)-methyl-3-(2-methoxy-4-dichlorophenyl)propionyl]piperazine::CHEMBL467587
SMILES COc1cc(Cl)ccc1C[C@@H](C)C(=O)N1CCN(CC1)c1ccc(Cl)cc1[C@@H](NC(=O)[C@@H](C)N)C(C)C
InChI Key InChIKey=BGNHNMCZDLGXJG-UDCNLSRBSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 4 hits for monomerid = 50261229
Found 4 hits for monomerid = 50261229
Affinity DataKi: 0.800nMAssay Description:Displacement of [125I]NDP-MSH from human MC4R expressed in HEK293 cellsMore data for this Ligand-Target Pair
Affinity DataKi: 34nMAssay Description:Displacement of [125I]NDP-MSH from human MC5R expressed in HEK293 cellsMore data for this Ligand-Target Pair
Affinity DataKi: 220nMAssay Description:Displacement of [125I]NDP-MSH from human MC3R expressed in HEK293 cellsMore data for this Ligand-Target Pair
Affinity DataIC50: 150nMAssay Description:Antagonist activity at human MC4R expressed in CHO cells assessed as inhibition of alpha-MSH-induced cAMP production by ELISAMore data for this Ligand-Target Pair