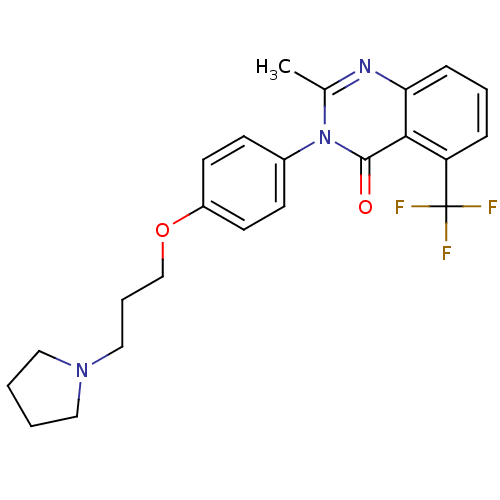
BDBM50262939 2-Methyl-3-(4-{[3-(1-pyrrolidinyl)propyl]oxy}phenyl)-5-(trifluoromethyl)-4(3H)-quinazolinone::CHEMBL476323
SMILES Cc1nc2cccc(c2c(=O)n1-c1ccc(OCCCN2CCCC2)cc1)C(F)(F)F
InChI Key InChIKey=DDDZBLNULGDPGA-UHFFFAOYSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 19 hits for monomerid = 50262939
Found 19 hits for monomerid = 50262939
Affinity DataKi: 0.531nMAssay Description:Displacement of [125I]Iodoproxyfan from human recombinant histamine H3 receptor by Competitive binding assayMore data for this Ligand-Target Pair
Affinity DataKi: 0.631nMAssay Description:Displacement of [3H]N-alpha-Methylhistamine from human recombinant H3 receptor expressed in HEK293 cells by competitive binding assayMore data for this Ligand-Target Pair
Affinity DataKi: 1.70nMAssay Description:Inhibition of human H3 receptorMore data for this Ligand-Target Pair
TargetHistamine receptor H3(Macaca mulatta (Rhesus macaque))
Tsukuba Research Institute
Curated by ChEMBL
Tsukuba Research Institute
Curated by ChEMBL
Affinity DataKi: 4.30nMAssay Description:Displacement of [3H]N-alpha-methylhistamine from rhesus monkey histamine H3 receptor expressed in HEK293T cellsMore data for this Ligand-Target Pair
Affinity DataKi: 6.80nMAssay Description:Displacement of [3H]N-alpha-methylhistamine from human histamine H3 receptor expressed in CHO-K1 cellsMore data for this Ligand-Target Pair
Affinity DataKi: 6.80nMAssay Description:Binding affinity to recombinant human H3 receptorMore data for this Ligand-Target Pair
Affinity DataKi: 33nMAssay Description:Displacement of [3H]N-alpha-methylhistamine from rat histamine H3 receptor expressed in HEK293 cells coexpressed with CRE-beta-lactamaseMore data for this Ligand-Target Pair
Affinity DataIC50: >2.70E+4nMAssay Description:Inhibition of CYP2C19More data for this Ligand-Target Pair
Affinity DataIC50: >2.70E+4nMAssay Description:Inhibition of CYP2D6More data for this Ligand-Target Pair
Affinity DataIC50: >2.70E+4nMAssay Description:Inhibition of CYP3A4More data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
Bioprojet-Biotech
Curated by ChEMBL
Bioprojet-Biotech
Curated by ChEMBL
Affinity DataIC50: 1.00E+4nMAssay Description:Displacement of [3H]dofetilide from human recombinant ERG by Competitive binding assayMore data for this Ligand-Target Pair
Affinity DataIC50: >2.10E+4nMAssay Description:Inhibition of CYP2C9More data for this Ligand-Target Pair
Affinity DataIC50: >2.70E+4nMAssay Description:Inhibition of CYP1A2More data for this Ligand-Target Pair
Affinity DataIC50: >1.00E+4nMAssay Description:Inhibition of human histamine H4 receptorMore data for this Ligand-Target Pair
Affinity DataIC50: >1.00E+4nMAssay Description:Inhibition of human histamine H2 receptorMore data for this Ligand-Target Pair
Affinity DataIC50: >1.00E+4nMAssay Description:Inhibition of human histamine H1 receptorMore data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
Bioprojet-Biotech
Curated by ChEMBL
Bioprojet-Biotech
Curated by ChEMBL
Affinity DataIC50: >1.00E+4nMAssay Description:Displacement of [35S]N-[(4R)-1'-[(2R)-6-cyano-1,2,3,4-tetrahydro-2-naphthalenyl]-3,4-dihydro-4-hydroxyspiro[2H-1-benzopyran-2,4'-piperidin]-6-yl]meth...More data for this Ligand-Target Pair
Affinity DataIC50: 1.70nMAssay Description:Displacement of [3H]N-alpha-methylhistamine from human histamine H3 receptor expressed in CHO-K1 cellsMore data for this Ligand-Target Pair
Affinity DataIC50: >2.70E+4nMAssay Description:Inhibition of CYP2A6More data for this Ligand-Target Pair