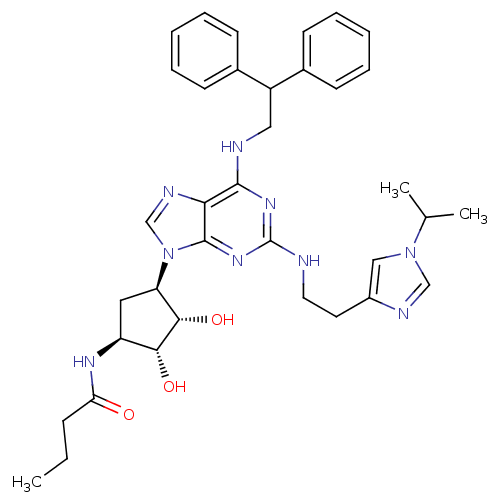
BDBM50309483 CHEMBL590718::N-((2S,3S,4R,5R)-5-(6-(2,2-diphenylethylamino)-2-(2-(1-isopropyl-1H-imidazol-4-yl)ethylamino)-9H-purin-9-yl)-3,4-dihydroxytetrahydrofuran-2-yl)butyramide
SMILES CCCC(=O)N[C@H]1C[C@H]([C@H](O)[C@@H]1O)n1cnc2c(NCC(c3ccccc3)c3ccccc3)nc(NCCc3cn(cn3)C(C)C)nc12
InChI Key InChIKey=CIMXZVURQFXWNX-MLVZTVGHSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 2 hits for monomerid = 50309483
Found 2 hits for monomerid = 50309483
TargetAdenosine receptor A2a(Homo sapiens (Human))
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Affinity DataKi: 39nMAssay Description:Displacement of [3H]NECA from human recombinant adenosine A2A receptor expressed in Sf21 cells co-expressing GalphaS2, beta4, gamma2More data for this Ligand-Target Pair
TargetAdenosine receptor A2a(Homo sapiens (Human))
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Affinity DataIC50: 160nMAssay Description:Agonist activity at adenosine A2A receptor in human neutrophils assessed as inhibition of fMLP-induced reactive oxygen species release by chemilumine...More data for this Ligand-Target Pair