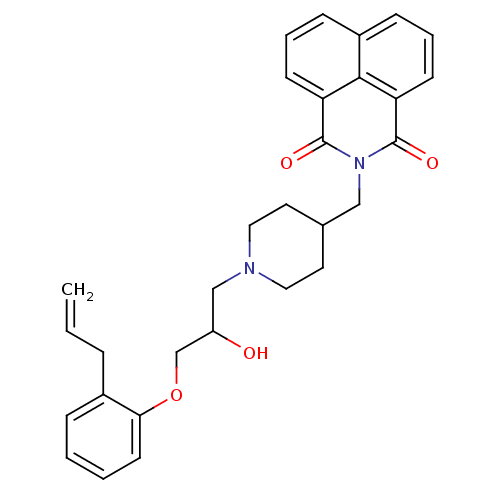
BDBM50318992 2-((1-(3-(2-allylphenoxy)-2-hydroxypropyl)piperidin-4-yl)methyl)-1H-benzo[de]isoquinoline-1,3(2H)-dione::CHEMBL1086005
SMILES OC(COc1ccccc1CC=C)CN1CCC(CN2C(=O)c3cccc4cccc(C2=O)c34)CC1
InChI Key InChIKey=XNVACWCCVJPZOD-UHFFFAOYSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 4 hits for monomerid = 50318992
Found 4 hits for monomerid = 50318992
Affinity DataKi: 80nMAssay Description:Displacement of [125I]cyanopindolol from human adrenergic beta2 receptor expressed in CHOK1 cellsMore data for this Ligand-Target Pair
Affinity DataKi: 80nMAssay Description:Antagonist activity at human adrenergic beta-1 receptor expressed in CHOK1 cells assessed as inhibition of cAMP accumulation by HTRF assayMore data for this Ligand-Target Pair
Affinity DataKi: 409nMAssay Description:Displacement of [125I]cyanopindolol from human adrenergic beta3 receptor expressed in CHOK1 cellsMore data for this Ligand-Target Pair
Affinity DataEC50: 2.40E+3nMAssay Description:Agonist activity at human adrenergic beta3 receptor expressed in CHOK1 cells assessed as cAMP accumulation by HTRF assayMore data for this Ligand-Target Pair