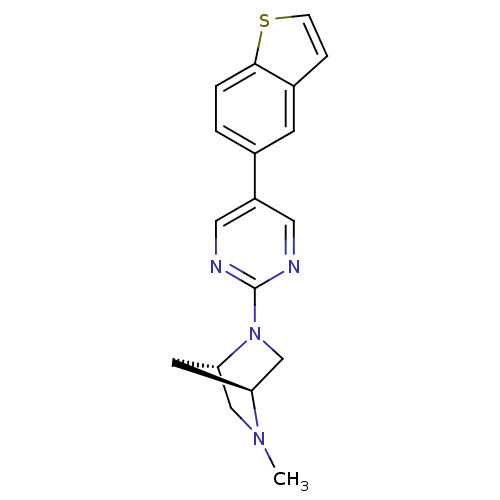
BDBM50319955 (1S,4S)-2-(5-(benzo[b]thiophen-5-yl)pyrimidin-2-yl)-5-methyl-2,5-diazabicyclo[2.2.1]heptane::CHEMBL1085806
SMILES CN1C[C@@H]2C[C@H]1CN2c1ncc(cn1)-c1ccc2sccc2c1
InChI Key InChIKey=GONGSLGOCPNPSS-HOTGVXAUSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 3 hits for monomerid = 50319955
Found 3 hits for monomerid = 50319955
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
Abbott Laboratories
Curated by ChEMBL
Abbott Laboratories
Curated by ChEMBL
Affinity DataKi: 0.560nMAssay Description:Displacement of [3H]dofetidile from human ERG by whole-cell patch clampMore data for this Ligand-Target Pair
TargetNeuronal acetylcholine receptor subunit alpha-7(Rattus norvegicus (Rat))
Abbott Laboratories
Curated by ChEMBL
Abbott Laboratories
Curated by ChEMBL
Affinity DataKi: 33nMAssay Description:Displacement of [3H]A585539 from alpha7 nACHR in rat brain homogenateMore data for this Ligand-Target Pair
TargetNeuronal acetylcholine receptor subunit alpha-7(Homo sapiens (Human))
Abbott Laboratories
Curated by ChEMBL
Abbott Laboratories
Curated by ChEMBL
Affinity DataEC50: 3.30E+4nMAssay Description:Agonist activity at human alpha7 nACHR expressed in Xenopus oocyte assessed as activation of current by voltage clamp methodMore data for this Ligand-Target Pair