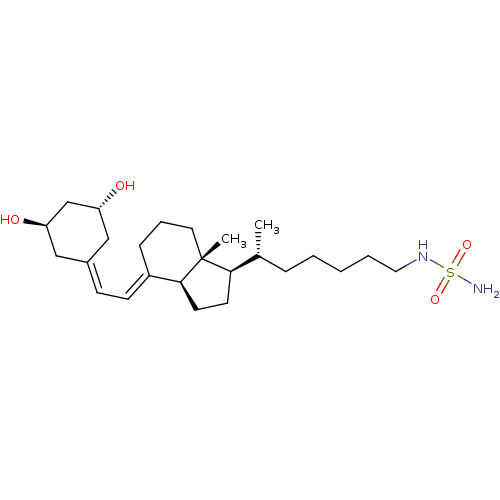
BDBM50320748 CHEMBL1164243::N-((R)-5-((1R,3aS,7aR,E)-4-(2-((3R,5R)-3,5-Dihydroxycycloheptylidene)ethylidene)-7a-methyloctahydro-1H-inden-1-yl)hexyl)methanesulfamide
SMILES [#6]-[#6@H](-[#6]-[#6]-[#6]-[#6]-[#6]-[#7]S([#7])(=O)=O)-[#6@H]1-[#6]-[#6]-[#6@H]2\[#6](-[#6]-[#6]-[#6][C@]12[#6])=[#6]\[#6]=[#6]-1/[#6]-[#6@@H](-[#8])-[#6]-[#6@H](-[#8])-[#6]-1
InChI Key InChIKey=LOCGBKCIQGOTKO-YLPIWGMZSA-N
Data 3 IC50
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 3 hits for monomerid = 50320748
Found 3 hits for monomerid = 50320748
Affinity DataIC50: 6.05E+5nMAssay Description:Inhibition of HDAC3 after 10 mins by fluorometric assayMore data for this Ligand-Target Pair
Affinity DataIC50: 196nMAssay Description:Binding affinity to VDR ligand binding domain by fluorescence polarization competition assayMore data for this Ligand-Target Pair
Affinity DataIC50: 3.16E+4nMAssay Description:Inhibition of HDAC6 after 10 mins by fluorometric assayMore data for this Ligand-Target Pair