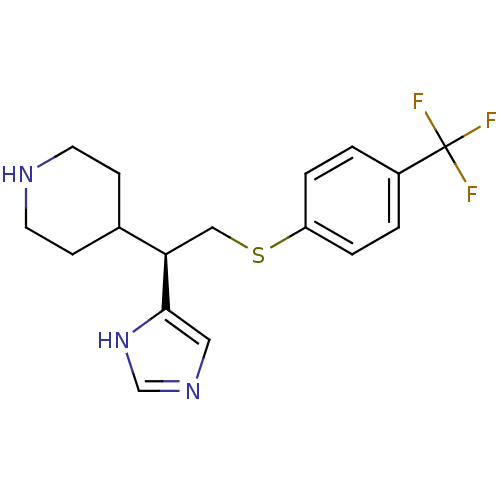
BDBM50326286 (R)-4-(1-(1H-Imidazol-4-yl)-2-(4-(trifluoromethyl)phenylthio)ethyl)piperidine::CHEMBL1243395
SMILES FC(F)(F)c1ccc(SC[C@H](C2CCNCC2)c2cnc[nH]2)cc1
InChI Key InChIKey=HGLGWLWXYRAXPK-OAHLLOKOSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 11 hits for monomerid = 50326286
Found 11 hits for monomerid = 50326286
Affinity DataKi: 18nMAssay Description:Displacement of [3H]-(R)-alpha-methylhistamine from human histamine H3 receptor expressed in CHO cells by liquid scintillation countingMore data for this Ligand-Target Pair
Affinity DataKi: 48nMAssay Description:Binding affinity to human histamine H1 receptorMore data for this Ligand-Target Pair
Affinity DataKi: 180nMAssay Description:Displacement of [3H]-(R)-alpha-methylhistamine from human histamine H3 receptor expressed in CHO cells by liquid scintillation countingMore data for this Ligand-Target Pair
Affinity DataKi: 310nMAssay Description:Binding affinity to human histamine H4 receptorMore data for this Ligand-Target Pair
Affinity DataKi: >1.00E+4nMAssay Description:Binding affinity to human histamine H2 receptorMore data for this Ligand-Target Pair
Affinity DataIC50: 9.50E+3nMAssay Description:Inhibition of CYP2D6More data for this Ligand-Target Pair
Affinity DataIC50: 1.20E+4nMAssay Description:Inhibition of CYP2C19More data for this Ligand-Target Pair
Affinity DataIC50: >9.40E+4nMAssay Description:Inhibition of CYP1A2More data for this Ligand-Target Pair
Affinity DataIC50: 1.70E+4nMAssay Description:Inhibition of CYP3A4More data for this Ligand-Target Pair
Affinity DataEC50: 38nMAssay Description:Agonist activity at human histamine H3 receptor expressed in CHO cells assessed as [35S]GTPgammaS bindingMore data for this Ligand-Target Pair
Affinity DataEC50: 74nMAssay Description:Agonist activity at human histamine H3 receptor expressed in CHO cells assessed as [35S]GTPgammaS bindingMore data for this Ligand-Target Pair