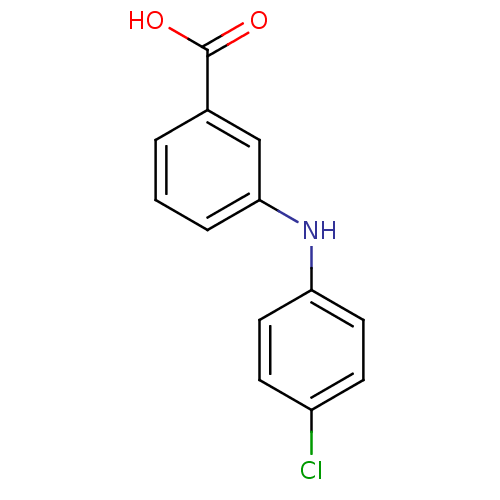
BDBM50337284 3-[N-(4-chlorophenyl)amino]benzoic acid::CHEMBL1682203::US9271961, 8
SMILES OC(=O)c1cccc(Nc2ccc(Cl)cc2)c1
InChI Key InChIKey=UPJCPFBYPSSQEM-UHFFFAOYSA-N
Data 11 IC50
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 11 hits for monomerid = 50337284
Found 11 hits for monomerid = 50337284
TargetAldo-keto reductase family 1 member C4(Homo sapiens (Human))
University Of Pennsylvania
US Patent
University Of Pennsylvania
US Patent
Affinity DataIC50: 4.87E+4nMAssay Description:Compounds may be evaluated as selective reversible inhibitors of AKR1C3 by screening them against homogeneous recombinant AKR1C1-AKR1C4 expressed in ...More data for this Ligand-Target Pair
TargetAldo-keto reductase family 1 member C3(Homo sapiens (Human))
University Of Pennsylvania
US Patent
University Of Pennsylvania
US Patent
Affinity DataIC50: 130nMAssay Description:Compounds may be evaluated as selective reversible inhibitors of AKR1C3 by screening them against homogeneous recombinant AKR1C1-AKR1C4 expressed in ...More data for this Ligand-Target Pair
TargetAldo-keto reductase family 1 member C2(Homo sapiens (Human))
University Of Pennsylvania
US Patent
University Of Pennsylvania
US Patent
Affinity DataIC50: 1.90E+4nMAssay Description:Compounds may be evaluated as selective reversible inhibitors of AKR1C3 by screening them against homogeneous recombinant AKR1C1-AKR1C4 expressed in ...More data for this Ligand-Target Pair
TargetAldo-keto reductase family 1 member C1(Homo sapiens (Human))
University Of Pennsylvania
US Patent
University Of Pennsylvania
US Patent
Affinity DataIC50: 3.02E+4nMAssay Description:Compounds may be evaluated as selective reversible inhibitors of AKR1C3 by screening them against homogeneous recombinant AKR1C1-AKR1C4 expressed in ...More data for this Ligand-Target Pair
Affinity DataIC50: >1.00E+5nMAssay Description:Compounds may be evaluated as selective reversible inhibitors of AKR1C3 by screening them against homogeneous recombinant AKR1C1-AKR1C4 expressed in ...More data for this Ligand-Target Pair
TargetAldo-keto reductase family 1 member C2(Homo sapiens (Human))
University Of Pennsylvania
US Patent
University Of Pennsylvania
US Patent
Affinity DataIC50: 1.91E+4nMAssay Description:Inhibition of recombinant AKR1C2 assessed as NADP+ dependent oxidation of S-tetralol by fluorescence assayMore data for this Ligand-Target Pair
TargetAldo-keto reductase family 1 member C2(Homo sapiens (Human))
University Of Pennsylvania
US Patent
University Of Pennsylvania
US Patent
Affinity DataIC50: 1.90E+4nMAssay Description:Inhibition of AKR1C2 by fluorimetric methodMore data for this Ligand-Target Pair
TargetAldo-keto reductase family 1 member C1(Homo sapiens (Human))
University Of Pennsylvania
US Patent
University Of Pennsylvania
US Patent
Affinity DataIC50: 3.02E+4nMAssay Description:Inhibition of recombinant AKR1C1 assessed as NADP+ dependent oxidation of S-tetralol by fluorescence assayMore data for this Ligand-Target Pair
TargetAldo-keto reductase family 1 member C4(Homo sapiens (Human))
University Of Pennsylvania
US Patent
University Of Pennsylvania
US Patent
Affinity DataIC50: 4.87E+4nMAssay Description:Inhibition of recombinant AKR1C4 assessed as NADP+ dependent oxidation of S-tetralol by fluorescence assayMore data for this Ligand-Target Pair
TargetAldo-keto reductase family 1 member C3(Homo sapiens (Human))
University Of Pennsylvania
US Patent
University Of Pennsylvania
US Patent
Affinity DataIC50: 140nMAssay Description:Inhibition of recombinant AKR1C3 assessed as NADP+ dependent oxidation of S-tetralol by fluorescence assayMore data for this Ligand-Target Pair
TargetAldo-keto reductase family 1 member C3(Homo sapiens (Human))
University Of Pennsylvania
US Patent
University Of Pennsylvania
US Patent
Affinity DataIC50: 130nMAssay Description:Inhibition of AKR1C3 by fluorimetric methodMore data for this Ligand-Target Pair