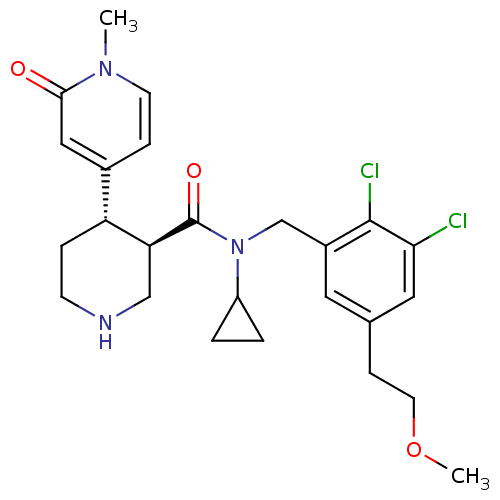
BDBM50346994 CHEMBL1795912
SMILES COCCc1cc(Cl)c(Cl)c(CN(C2CC2)C(=O)[C@H]2CNCC[C@@H]2c2ccn(C)c(=O)c2)c1
InChI Key InChIKey=VWRLHSUMSWLCDO-RTWAWAEBSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 3 hits for monomerid = 50346994
Found 3 hits for monomerid = 50346994
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
Merck Frosst Centre For Therapeutic Research
Curated by ChEMBL
Merck Frosst Centre For Therapeutic Research
Curated by ChEMBL
Affinity DataKi: 1.10E+4nMAssay Description:Inhibition of human ERGMore data for this Ligand-Target Pair
Affinity DataIC50: 2.20nMAssay Description:Inhibition of renin in human plasma using Q-FRET substrate pretreated for 10 mins before substrate addition measured after 1 hrMore data for this Ligand-Target Pair
Affinity DataIC50: 0.600nMAssay Description:Inhibition of renin in human plasma using Q-FRET substrate pretreated for 10 mins before substrate addition measured after 1 hrMore data for this Ligand-Target Pair