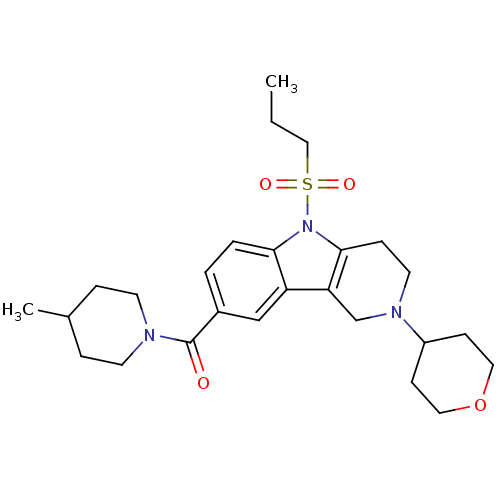
BDBM50364931 CHEMBL1950346
SMILES CCCS(=O)(=O)n1c2CCN(Cc2c2cc(ccc12)C(=O)N1CCC(C)CC1)C1CCOCC1
InChI Key InChIKey=LCLHWAFOYICJIC-UHFFFAOYSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 3 hits for monomerid = 50364931
Found 3 hits for monomerid = 50364931
Affinity DataKi: 115nMAssay Description:Displacement of [3H]-CP55,940 from human CB1 receptor expressed in HEK293 cellsMore data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
Astrazeneca R&D Montreal
Curated by ChEMBL
Astrazeneca R&D Montreal
Curated by ChEMBL
Affinity DataIC50: 2.00E+3nMAssay Description:Inhibition of human Erg by voltage ion flux electrophysiological assayMore data for this Ligand-Target Pair
Affinity DataEC50: 129nMAssay Description:Agonist activity at human CB1 receptor expressed in HEK293 EBNA cells by [35S]GTPgamma binding assayMore data for this Ligand-Target Pair