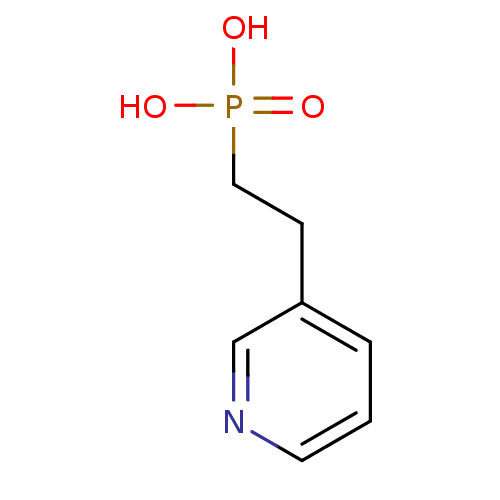
BDBM50373092 CHEMBL261311
SMILES OP(O)(=O)CCc1cccnc1
InChI Key InChIKey=VHGPDNZJZXWUDY-UHFFFAOYSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 4 hits for monomerid = 50373092
Found 4 hits for monomerid = 50373092
Affinity DataKi: 1.28E+5nMAssay Description:Inhibition of human recombinant FPPS expressed in Escherichia coli BL21 after 10 minsMore data for this Ligand-Target Pair
Affinity DataIC50: 8.24E+5nMAssay Description:Inhibition of human recombinant FPPS expressed in Escherichia coli BL21 after 10 minsMore data for this Ligand-Target Pair
Affinity DataIC50: 200nMAssay Description:Inhibition of FPPS (unknown origin)More data for this Ligand-Target Pair
Affinity DataIC50: >8.00E+5nMAssay Description:Allosteric inhibition of human recombinant FPPS using GPP and [3H]IPP as substrate incubated with enzyme for 10 mins prior to substrate addition by l...More data for this Ligand-Target Pair