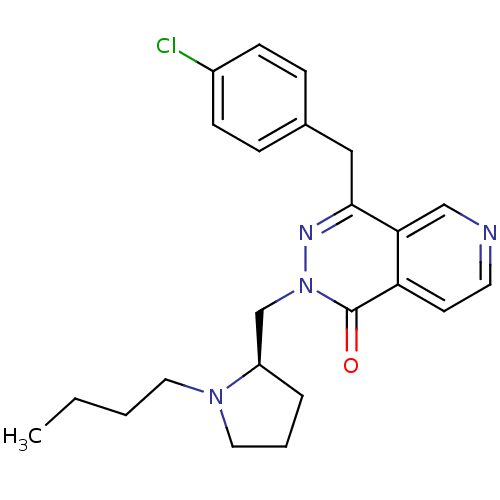
BDBM50391697 CHEMBL2146806
SMILES CCCCN1CCC[C@@H]1Cn1nc(Cc2ccc(Cl)cc2)c2cnccc2c1=O
InChI Key InChIKey=VFIDGXZRONKQBZ-LJQANCHMSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 5 hits for monomerid = 50391697
Found 5 hits for monomerid = 50391697
Affinity DataKi: 0.398nMAssay Description:Antagonist activity at human H1 receptor expressed in CHO cells assessed as inhibition of calcium mobilization by FLIPR assayMore data for this Ligand-Target Pair
Affinity DataKi: 6.31nMAssay Description:Antagonist activity at human adrenergic alpha1A receptor expressed in rat fibroblasts by by plate-based calcium imagingMore data for this Ligand-Target Pair
Affinity DataKi: 7.94nMAssay Description:Antagonist activity at human adrenergic alpha1B receptor expressed in rat fibroblasts by by plate-based calcium imagingMore data for this Ligand-Target Pair
Affinity DataKi: 631nMAssay Description:Antagonist activity at human H3 receptor expressed in CHO cells assessed as inhibition of histamine-induced GTPgamma[S] binding by scintillation prox...More data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
Glaxosmithkline
Curated by ChEMBL
Glaxosmithkline
Curated by ChEMBL
Affinity DataIC50: 50.1nMAssay Description:Displacement of [3H]dofetilide from human ERGMore data for this Ligand-Target Pair