BDBM50524986 CHEMBL4516124
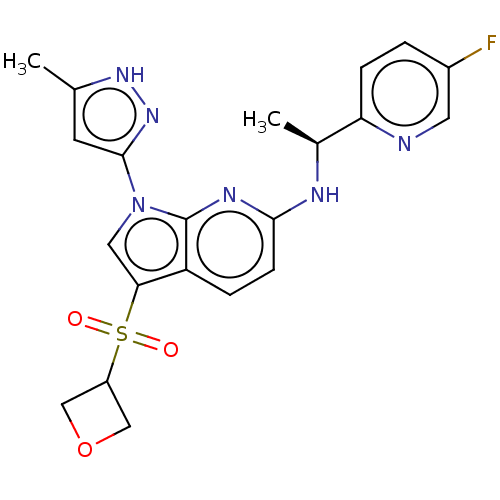
SMILES C[C@H](Nc1ccc2c(cn(-c3cc(C)[nH]n3)c2n1)S(=O)(=O)C1COC1)c1ccc(F)cn1
InChI Key InChIKey=NNWRFTLSEJKLQI-ZDUSSCGKSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 3 hits for monomerid = 50524986
Found 3 hits for monomerid = 50524986
TargetHigh affinity nerve growth factor receptor(Homo sapiens (Human))
Takeda Pharmaceutical
Curated by ChEMBL
Takeda Pharmaceutical
Curated by ChEMBL
Affinity DataIC50: 730nMAssay Description:Inhibition of N-terminal GST-tagged human TrkA kinase domain (436 to 790 residues) expressed in baculovirus expression system using biotin-poly-GT as...More data for this Ligand-Target Pair
Affinity DataIC50: 60nMAssay Description:Inhibition of recombinant human ALK expressed in HEK293 cells assessed as reduction in ALK autophosphorylation at Tyr1604 residue incubated for 60 mi...More data for this Ligand-Target Pair
Affinity DataIC50: 20nMAssay Description:Inhibition of human ALK kinase domain (1058 to 1620 residues) expressed in baculovirus expression system using biotin-poly-GT as substrate pre-incuba...More data for this Ligand-Target Pair
 3D Structure (crystal)
3D Structure (crystal)