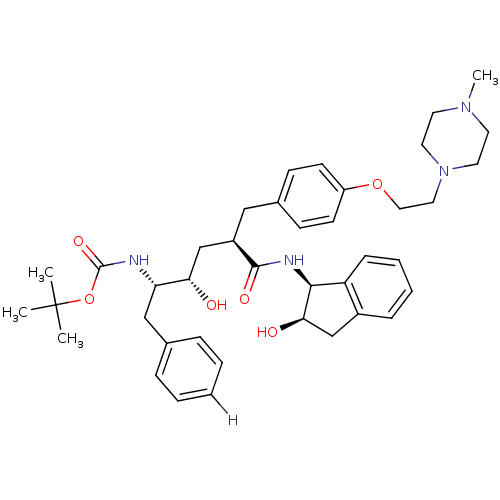
BDBM888 L-685,434 deriv. 36::N-(2(R)-Hydroxy-1(S)-indanyl)-5(S)-[(tert-butyloxycarbonyl)amino]-4(S)-hydroxy-6-phenyl-2(R)-[[4-[2-(4-methylpiperazin-1-yl)ethoxy]phenyl]methyl]hexanamide::tert-butyl N-[(2S,3S,5R)-3-hydroxy-5-{[(1S,2R)-2-hydroxy-2,3-dihydro-1H-inden-1-yl]carbamoyl}-5-({4-[2-(4-methylpiperazin-1-yl)ethoxy]phenyl}methyl)-1-phenylpentan-2-yl]carbamate
SMILES CN1CCN(CCOc2ccc(C[C@H](C[C@H](O)[C@H](Cc3ccccc3)NC(=O)OC(C)(C)C)C(=O)N[C@@H]3[C@H](O)Cc4ccccc34)cc2)CC1
InChI Key InChIKey=XJMAXQMSDUBZFJ-SZNOJMITSA-N
Data 1 IC50
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 1 hit for monomerid = 888
Found 1 hit for monomerid = 888
TargetDimer of Gag-Pol polyprotein [489-587](Human immunodeficiency virus type 1)
Merck Research Laboratories
Merck Research Laboratories
Affinity DataIC50: 0.200nMpH: 5.5 T: 2°CAssay Description:Sensitivity of HIV-1 protease activity to protease inhibitors was determined by a peptide substrate cleavage assay. Protease products were analyzed o...More data for this Ligand-Target Pair