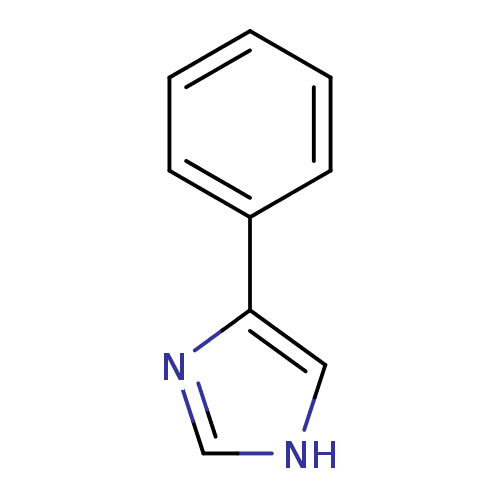
BDBM24656 4-PIM::4-Phenylimidazole::4-phenyl-1H-imidazole::4-phenylimidazole, 4-PI::CHEMBL14145::US9138393, 4-Phenyl- imidazole::US9144538, 4-Phenylimidazole
SMILES c1nc(c[nH]1)-c1ccccc1
InChI Key InChIKey=XHLKOHSAWQPOFO-UHFFFAOYSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 19 hits for monomerid = 24656
Found 19 hits for monomerid = 24656
Affinity DataIC50: 4.80E+4nMpH: 6.5 T: 2°CAssay Description:The IC50 inhibition assays were performed in a 96-well microtiter plate format using purified recombinant IDO, which was added to the substrate, L-tr...More data for this Ligand-Target Pair
Affinity DataIC50: 4.80E+4nMAssay Description:Inhibition of human IDO1 expressed in Escherichia coli BL21 (DE3) using L-tryptophan as substrateMore data for this Ligand-Target Pair
Affinity DataKd: 2.80E+5nMAssay Description:Substrate and ligand binding assay using UV- visible absorbance analysis of CYP142 was done on a Cary UV-50 UV-visible scanning spectrophotometer (Va...More data for this Ligand-Target Pair
Affinity DataKd: 2.16E+5nMAssay Description:Substrate and ligand binding assay using UV- visible absorbance analysis of CYP142 was done on a Cary UV-50 UV-visible scanning spectrophotometer (Va...More data for this Ligand-Target Pair
Affinity DataKd: 1.20E+4nMAssay Description:Substrate and ligand binding assay using UV- visible absorbance analysis of CYP142 was done on a Cary UV-50 UV-visible scanning spectrophotometer (Va...More data for this Ligand-Target Pair
Affinity DataIC50: 5.88E+4nMpH: 7.4 T: 2°CAssay Description:Microsomal preparation: One lobe of fresh pig liver is obtained (e.g., at about the time of slaughter from a food-processing company) and immediately...More data for this Ligand-Target Pair
Affinity DataIC50: 1.50E+3nMAssay Description:A commercially available P450-GLO Assay kit (Promega Corporation, Madison Wis.) is used to screen various compounds for CYP3A4A inhibition activity. ...More data for this Ligand-Target Pair
Affinity DataIC50: 5.88E+4nMpH: 7.4Assay Description:All procedures were carried out under minimal light in order to prevent degradation of the retinoid samples.Microsomal preparation: one lobe of fresh...More data for this Ligand-Target Pair
Affinity DataIC50: 1.50E+3nMAssay Description:Cytochrome P450 is a large and diverse group of enzymes that catalyze the oxidation of organic substances. Some members of the CYP family contribute ...More data for this Ligand-Target Pair
Affinity DataKd: 2.98E+6nMpH: 7.0Assay Description:Optical titrations to determine the binding affinity (KD values) of ligands were carried out on a Cary 60 UV−visible spectrophotometer (Varian,...More data for this Ligand-Target Pair
TargetSerine/threonine-protein kinase mTOR(Homo sapiens (Human))
Abbott Laboratories
Curated by ChEMBL
Abbott Laboratories
Curated by ChEMBL
Affinity DataKd: >1.00E+7nMAssay Description:Dissociation constant when binding to FK506 binding protein (FKBP).More data for this Ligand-Target Pair
Affinity DataIC50: 3.30E+4nMAssay Description:Inhibition of Aryl hydrocarbon hydroxylase in phenobarbitone-treated ratsMore data for this Ligand-Target Pair
Affinity DataIC50: 7.03E+4nMAssay Description:Inhibition of human recombinant N-terminus 6X-histidine-tagged indoleamine 2,3-dioxygenase expressed in Escherichia coli assessed as inhibition of L-...More data for this Ligand-Target Pair
Affinity DataIC50: 7.40E+3nMAssay Description:Inhibition of Aminopyrine N-demethylase in Phenobarbitone-treated ratsMore data for this Ligand-Target Pair
Affinity DataIC50: 3.10E+4nMAssay Description:Inhibition of recombinant human IDO1 expressed in bacterial expression system using L-Tryptophan as substrate after 25 mins in absence of GSH and twe...More data for this Ligand-Target Pair
Affinity DataIC50: 3.80E+4nMAssay Description:Inhibition of recombinant human IDO1 expressed in bacterial expression system using L-Tryptophan as substrate after 25 mins in absence of GSH and pre...More data for this Ligand-Target Pair
Affinity DataIC50: 4.28E+5nMAssay Description:Inhibition of full length recombinant human His-tagged IDO1 expressed in mouse LLTC cells using L-tryptophan as substrate after 24 hrsMore data for this Ligand-Target Pair
Affinity DataIC50: 4.20E+4nMAssay Description:Inhibition of recombinant human IDO1 expressed in bacterial expression system using L-Tryptophan as substrate after 25 mins in presence of GSH and tw...More data for this Ligand-Target Pair
TargetLanosterol 14-alpha demethylase(Mycobacterium tuberculosis (strain CDC 1551 / Oshk...)
University Of California San Francisco
University Of California San Francisco
Affinity DataKd: 1.30E+6nMpH: 7.5 T: 2°CAssay Description:The assay was developed based on the optical spectral property of P450 enzyme to elicit both type I and type II spectral changes (350 nm through 450 ...More data for this Ligand-Target Pair