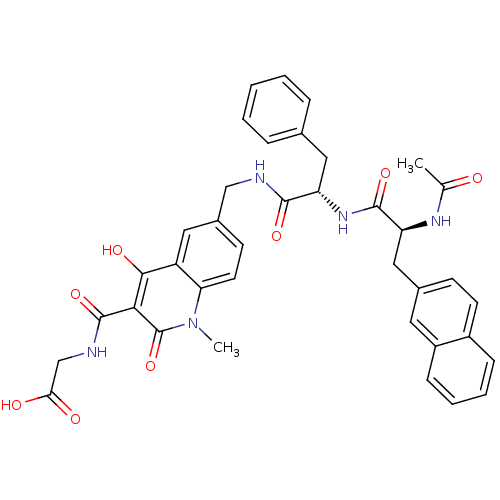
BDBM93422 PHD Inhibitor, 12{1,1,2}
SMILES CC(=O)N[C@@H](Cc1ccc2ccccc2c1)C(=O)N[C@@H](Cc1ccccc1)C(=O)NCc1ccc2n(C)c(=O)c(C(=O)NCC(O)=O)c(O)c2c1
InChI Key InChIKey=STTWGTPJKOYMRB-KYJUHHDHSA-N
Data 3 IC50
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 3 hits for monomerid = 93422
Found 3 hits for monomerid = 93422
Affinity DataIC50: 35nMAssay Description:PHD1,2,3 activity was measured utilizing homogeneous time-resolved fluroescence energy transfer techology by detecting the trans-4-hydroxylatio of HI...More data for this Ligand-Target Pair
Affinity DataIC50: 4.5nMAssay Description:PHD1,2,3 activity was measured utilizing homogeneous time-resolved fluroescence energy transfer techology by detecting the trans-4-hydroxylatio of HI...More data for this Ligand-Target Pair
Affinity DataIC50: 4.30nMAssay Description:PHD1,2,3 activity was measured utilizing homogeneous time-resolved fluroescence energy transfer techology by detecting the trans-4-hydroxylatio of HI...More data for this Ligand-Target Pair