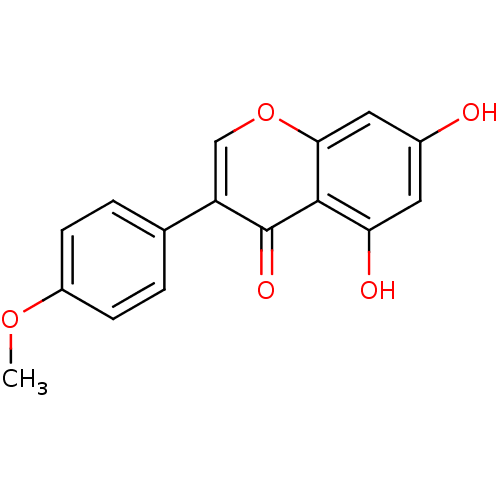
BDBM9461 5,7-dihydroxy-3-(4-methoxyphenyl)-4H-chromen-4-one::Biochanin A (2)::Biochanin A (BCA)::Biochanin A, 9::CHEMBL131921::cid_5280373
SMILES COc1ccc(cc1)-c1coc2cc(O)cc(O)c2c1=O
InChI Key InChIKey=WUADCCWRTIWANL-UHFFFAOYSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 30 hits for monomerid = 9461
Found 30 hits for monomerid = 9461
TargetCarbonic anhydrase 12(Homo sapiens (Human))
Aristotle University Of Thessaloniki
Curated by ChEMBL
Aristotle University Of Thessaloniki
Curated by ChEMBL
Affinity DataKi: 53nM ΔG°: -9.92kcal/moleT: 2°CAssay Description:Inhibition of human recombinant carbonic anhydrase 12 preincubated for 15 mins at room temperature/6 hrs at 4 deg C by stopped-flow CO2 hydration ass...More data for this Ligand-Target Pair
TargetCarbonic anhydrase 7(Homo sapiens (Human))
Aristotle University Of Thessaloniki
Curated by ChEMBL
Aristotle University Of Thessaloniki
Curated by ChEMBL
Affinity DataKi: 372nM ΔG°: -8.77kcal/moleT: 2°CAssay Description:Inhibition of human recombinant carbonic anhydrase 7 preincubated for 15 mins at room temperature/6 hrs at 4 deg C by stopped-flow CO2 hydration assa...More data for this Ligand-Target Pair
Affinity DataKi: 1.90E+3nMAssay Description:Inhibition of CYP1B1 in human liver microsomes coexpressing recombinant human cytochrome P450 oxidoreductase using 7-ethoxyresorufin as substrate aft...More data for this Ligand-Target Pair
Affinity DataKi: 2.03E+3nMAssay Description:Binding affinity to human recombinant carbonyl reductase 1 expressed in Escherichia coli assessed as NADPH oxidation using isatin as substrateMore data for this Ligand-Target Pair
TargetCarbonic anhydrase 4(Homo sapiens (Human))
Aristotle University Of Thessaloniki
Curated by ChEMBL
Aristotle University Of Thessaloniki
Curated by ChEMBL
Affinity DataKi: 7.08E+3nM ΔG°: -7.02kcal/moleT: 2°CAssay Description:Inhibition of human recombinant carbonic anhydrase 4 preincubated for 15 mins at room temperature/6 hrs at 4 deg C by stopped-flow CO2 hydration assa...More data for this Ligand-Target Pair
TargetCarbonic anhydrase 1(Homo sapiens (Human))
Aristotle University Of Thessaloniki
Curated by ChEMBL
Aristotle University Of Thessaloniki
Curated by ChEMBL
Affinity DataKi: >1.00E+4nM ΔG°: >-6.82kcal/moleT: 2°CAssay Description:Inhibition of human recombinant carbonic anhydrase 1 preincubated for 15 mins at room temperature/6 hrs at 4 deg C by stopped-flow CO2 hydration assa...More data for this Ligand-Target Pair
TargetCarbonic anhydrase 2(Homo sapiens (Human))
Aristotle University Of Thessaloniki
Curated by ChEMBL
Aristotle University Of Thessaloniki
Curated by ChEMBL
Affinity DataKi: >1.00E+4nM ΔG°: >-6.82kcal/moleT: 2°CAssay Description:Inhibition of human recombinant carbonic anhydrase 2 preincubated for 15 mins at room temperature/6 hrs at 4 deg C by stopped-flow CO2 hydration assa...More data for this Ligand-Target Pair
Affinity DataKi: 1.20E+4nM ΔG°: -6.98kcal/mole IC50: 3.40E+4nMpH: 7.0 T: 2°CAssay Description:Inhibition of human placental aromatase was determined by monitoring the amount of 3H2O released as the enzyme converts [1beta-3H]androst-4-ene-3,17-...More data for this Ligand-Target Pair
Affinity DataKi: 1.20E+4nM ΔG°: -6.98kcal/mole IC50: 3.40E+4nMpH: 7.0 T: 2°CAssay Description:Inhibition of human placental aromatase was determined by monitoring the amount of 3H2O released as the enzyme converts [1beta-3H]androst-4-ene-3,17-...More data for this Ligand-Target Pair
Affinity DataKi: 1.20E+4nMAssay Description:Inhibition of human placental microsome aromatase using [1beta,2beta3H]androstenedione as substrate after 15 mins in presence of NADPH by liquid scin...More data for this Ligand-Target Pair
Affinity DataIC50: 1.05E+5nMAssay Description:Inhibition of EGFR in human A431 cellsMore data for this Ligand-Target Pair
TargetPancreatic triacylglycerol lipase(Sus scrofa (Pig))
Chungbuk National University
Curated by ChEMBL
Chungbuk National University
Curated by ChEMBL
Affinity DataIC50: >1.00E+5nMAssay Description:Inhibition of porcine pancreatic lipase pre-incubated for 15 mins before p-nitrophenylbutyrate substrate addition by microplate reader based methodMore data for this Ligand-Target Pair
Affinity DataIC50: 1.08E+4nMpH: 7.45 T: 2°CAssay Description:Inhibition assay of human testes mcrosomal 17 beta-hydroxysteroid dehydrogenase for the reduction of androstenedione.More data for this Ligand-Target Pair
Affinity DataEC50: 5.59E+4nMAssay Description:Southern Research Molecular Libraries Screening Center (SRMLSC) Southern Research Institute (Birmingham, Alabama) NIH Molecular Libraries Screening C...More data for this Ligand-Target Pair
TargetD(1B) dopamine receptor(Homo sapiens (Human))
Vanderbilt Screening Center For Gpcrs, Ion Channels And Transporters
Curated by PubChem BioAssay
Vanderbilt Screening Center For Gpcrs, Ion Channels And Transporters
Curated by PubChem BioAssay
Affinity DataEC50: 0.00334nMAssay Description:Assay Provider: Val Watts Assay Provider Affiliation: Purdue University Grant Title: Allosteric Modulators of D1 Receptors Grant Number: 1 X01 MH0776...More data for this Ligand-Target Pair
Affinity DataIC50: 1.27E+4nMAssay Description:Binding affinity to human recombinant carbonyl reductase 1 expressed in Escherichia coli assessed as NADPH oxidation using isatin as substrateMore data for this Ligand-Target Pair
TargetSignal transducer and activator of transcription 3 [702-738,740-752](Homo sapiens (Human))
The Scripps Research Institute Molecular Screening Center
Curated by PubChem BioAssay
The Scripps Research Institute Molecular Screening Center
Curated by PubChem BioAssay
Affinity DataEC50: >5.57E+4nMAssay Description:Source (MLPCN Center Name): The Scripps Research Institute Molecular Screening Center Center Affiliation: The Scripps Research Institute (TSRI) Assay...More data for this Ligand-Target Pair
Affinity DataIC50: 1.60E+6nMAssay Description:Antagonist activity at androgen receptor in human MDA-kb2 cells assessed as inhibition of DHT-induced luciferase activity by luciferase reporter gene...More data for this Ligand-Target Pair
Target3-hydroxyacyl-[acyl-carrier-protein] dehydratase(Plasmodium falciparum)
University Of Zurich
Curated by ChEMBL
University Of Zurich
Curated by ChEMBL
Affinity DataIC50: 8.00E+3nMAssay Description:Inhibition of FabZMore data for this Ligand-Target Pair
Affinity DataIC50: 10.2nMAssay Description:Inhibition of human placental microsome CYP19More data for this Ligand-Target Pair
Target17-beta-hydroxysteroid dehydrogenase type 2(Homo sapiens (Human))
University Of Innsbruck
Curated by ChEMBL
University Of Innsbruck
Curated by ChEMBL
Affinity DataIC50: 9.90E+3nMAssay Description:Inhibition of human recombinant 17beta-HSD2 expressed in HEK293 cell lysate assessed as conversion of radiolabeled estrone to estradiolMore data for this Ligand-Target Pair
Affinity DataIC50: 1.13E+5nMAssay Description:Inhibition of aromataseMore data for this Ligand-Target Pair
TargetLysine-specific histone demethylase 1A(Homo sapiens (Human))
Hangzhou Normal University
Curated by ChEMBL
Hangzhou Normal University
Curated by ChEMBL
Affinity DataIC50: 2.95E+3nMAssay Description:Inhibition of recombinant KDM1A (unknown origin) using H3K4Me2 peptide as substrate incubated for 0.5 hrs by Amplex red assayMore data for this Ligand-Target Pair
Target17-beta-hydroxysteroid dehydrogenase type 2(Homo sapiens (Human))
University Of Innsbruck
Curated by ChEMBL
University Of Innsbruck
Curated by ChEMBL
Affinity DataIC50: 9.90E+3nMAssay Description:Inhibition of human 17beta-HSD2 expressed in HEK293 cell lysates incubated for 10 mins using [2,4,6,7-3H]-estradiol and NAD+ by scintillation countin...More data for this Ligand-Target Pair
TargetAldo-keto reductase family 1 member B1(Homo sapiens (Human))
University Of Hradec Kralove
Curated by ChEMBL
University Of Hradec Kralove
Curated by ChEMBL
Affinity DataIC50: 1.27E+5nMAssay Description:Inhibition of recombinant N-terminal His6-tagged AKR1B1 (unknown origin) expressed in Escherichia coli BL21 cells using all-trans-retinal as substrat...More data for this Ligand-Target Pair
TargetAldo-keto reductase family 1 member B10(Homo sapiens (Human))
University Of Hradec Kralove
Curated by ChEMBL
University Of Hradec Kralove
Curated by ChEMBL
Affinity DataIC50: 3.61E+4nMAssay Description:Inhibition of recombinant human N-terminal His6-tagged AKR1B10 expressed in Escherichia coli BL21 cells using all-trans-retinal as substrate incubate...More data for this Ligand-Target Pair
TargetLysine-specific histone demethylase 1A(Homo sapiens (Human))
Hangzhou Normal University
Curated by ChEMBL
Hangzhou Normal University
Curated by ChEMBL
Affinity DataIC50: 2.95E+3nMAssay Description:Inhibition of recombinant LSD1 (unknown origin) using H3K4me2 as a substrate measured after 0.5 hrs by fluorescence based analysisMore data for this Ligand-Target Pair
TargetLysine-specific histone demethylase 1A(Homo sapiens (Human))
Hangzhou Normal University
Curated by ChEMBL
Hangzhou Normal University
Curated by ChEMBL
Affinity DataIC50: 2.95E+3nMAssay Description:Inhibition of recombinant LSD1 (unknown origin) incubated for 1 hrMore data for this Ligand-Target Pair
Affinity DataIC50: 1.13E+5nMAssay Description:Inhibition of aromatase (unknown origin)More data for this Ligand-Target Pair
Affinity DataIC50: 1.00E+5nMpH: 8.0 T: 2°CAssay Description:A mixture of test compound (10 μL; 1 mM) in MeOH, type-1B lipoxygenase (EC 1.13.11.12; from soyabean) (20 μL; 70 units) in 0.1 M aqueous ph...More data for this Ligand-Target Pair