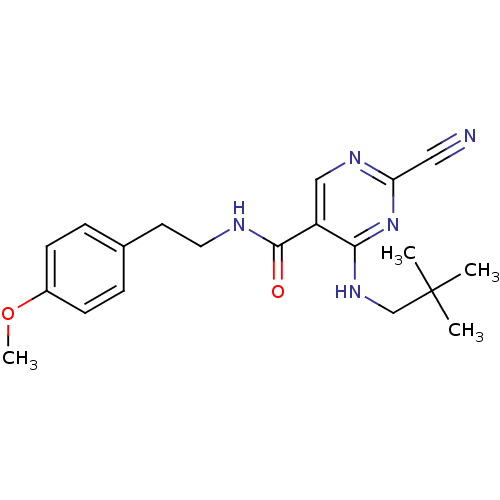
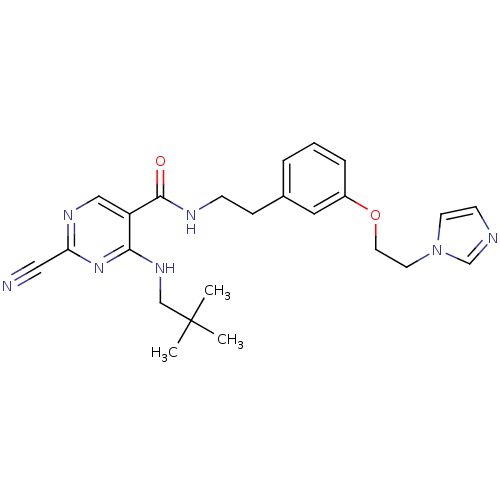
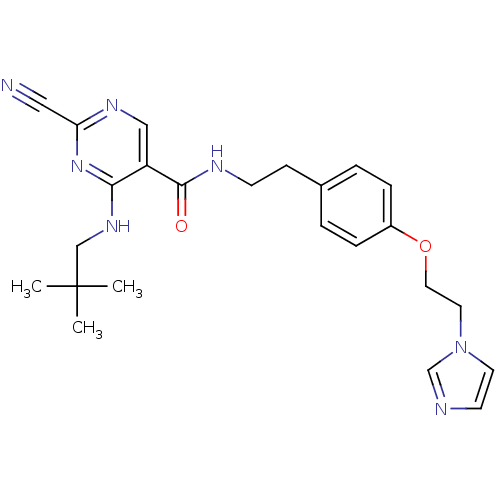
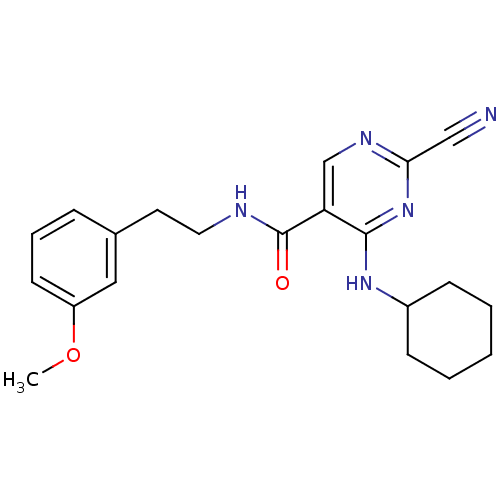
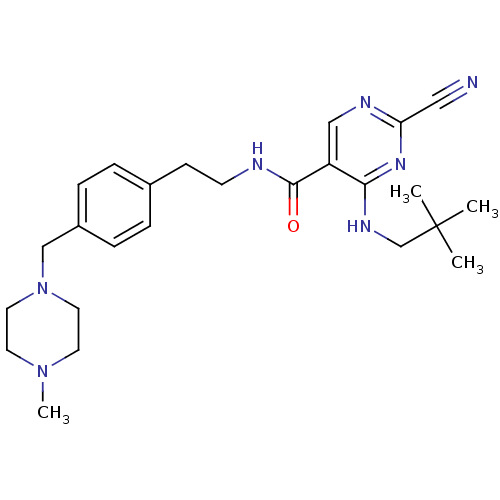
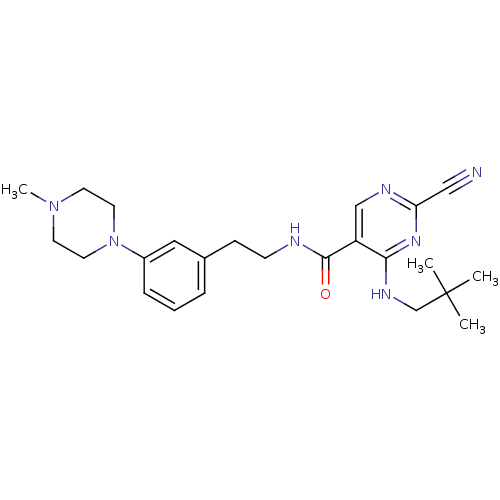
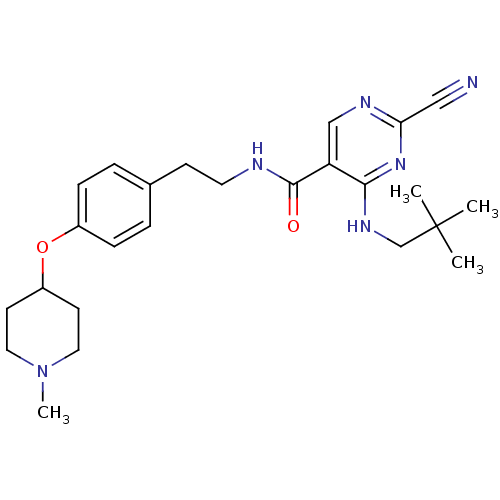
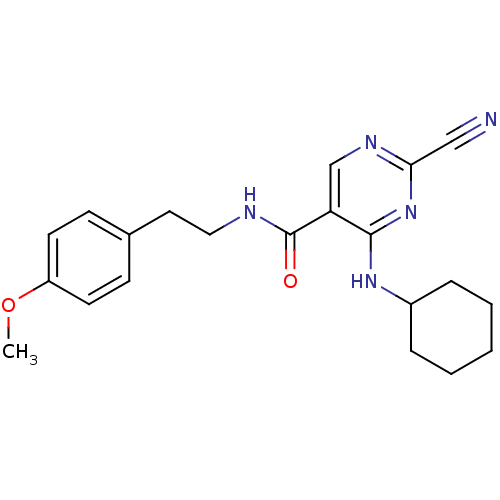
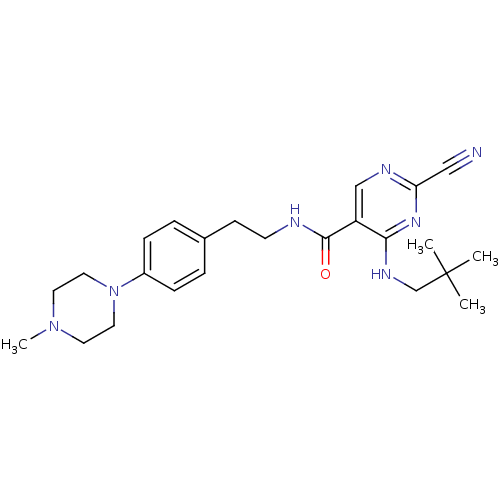
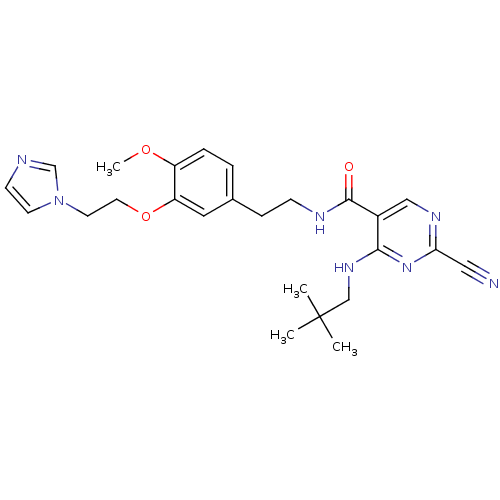
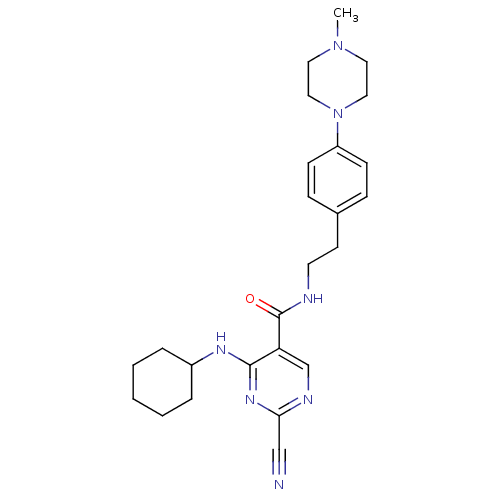
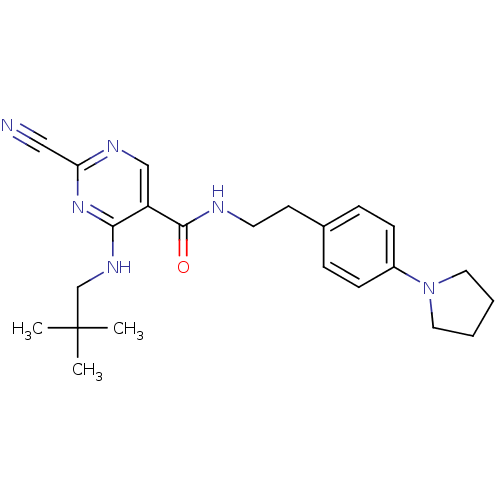
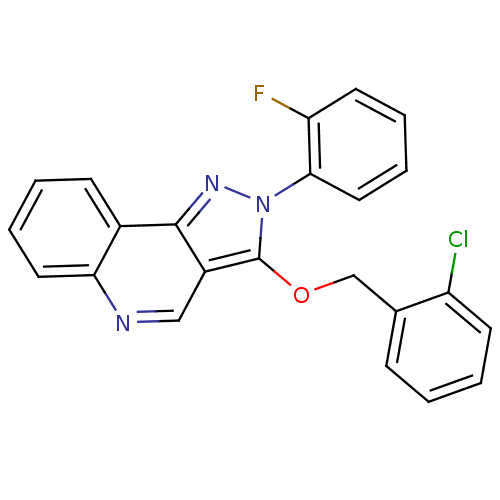
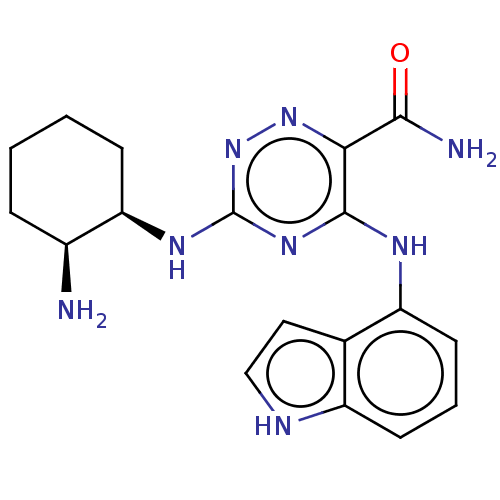
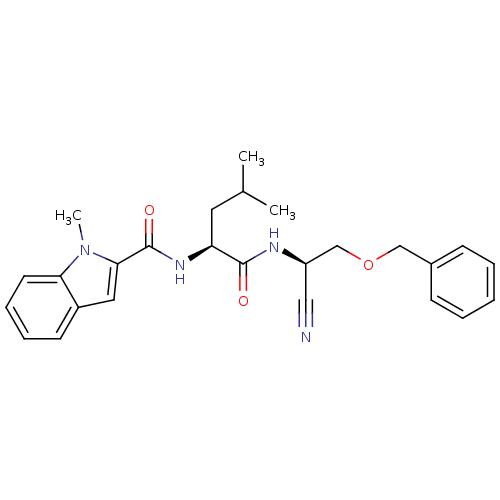
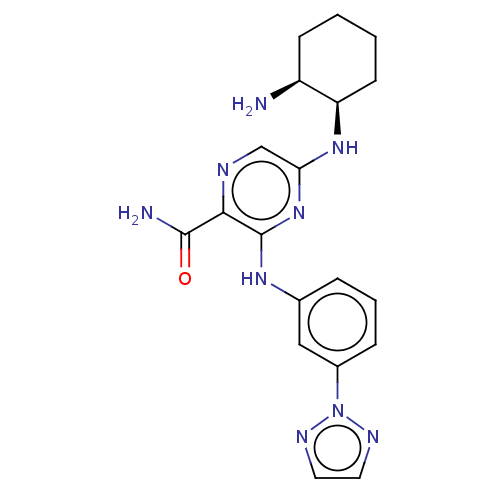
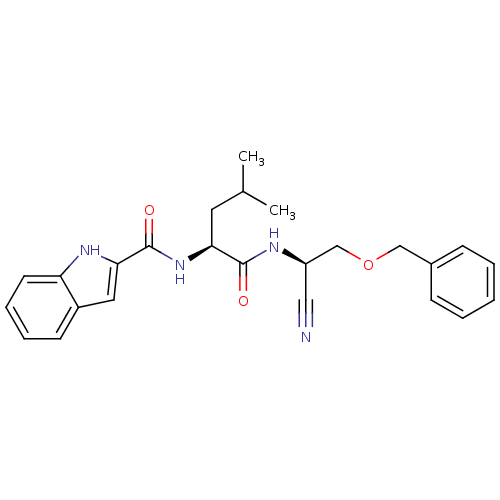
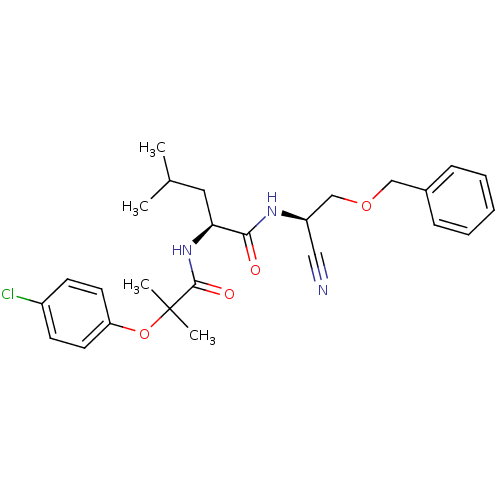
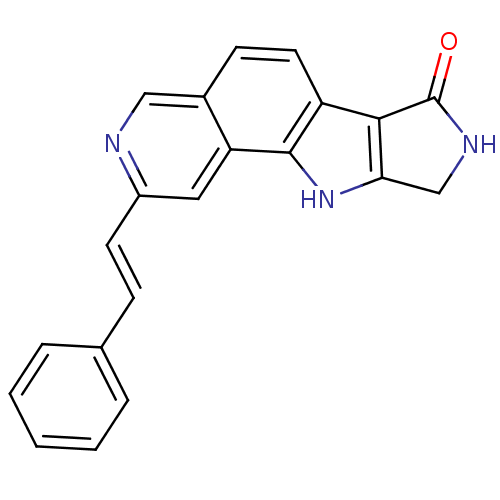
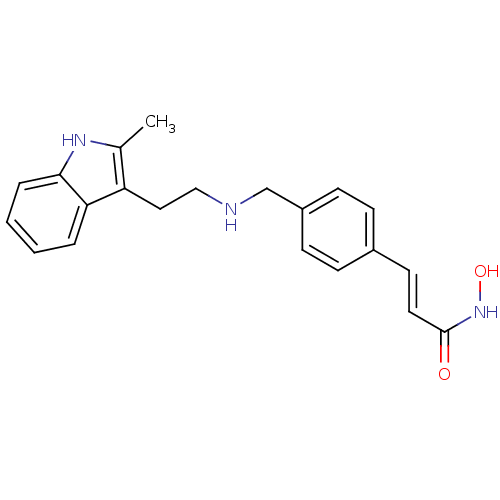
Affinity DataIC50: <0.00300nMpH: 7.0 T: 2°CAssay Description:The substrate hydrolysis with or without inhibitor was monitored at an excitation wavelength of 360nm and an emission wavelength of 460 nm on a fluor...More data for this Ligand-Target Pair
Affinity DataIC50: <0.00300nMpH: 7.0 T: 2°CAssay Description:The substrate hydrolysis with or without inhibitor was monitored at an excitation wavelength of 360nm and an emission wavelength of 460 nm on a fluor...More data for this Ligand-Target Pair
Affinity DataIC50: 0.00300nMpH: 7.0 T: 2°CAssay Description:The substrate hydrolysis with or without inhibitor was monitored at an excitation wavelength of 360nm and an emission wavelength of 460 nm on a fluor...More data for this Ligand-Target Pair
Affinity DataIC50: 0.00300nMpH: 7.0 T: 2°CAssay Description:The substrate hydrolysis with or without inhibitor was monitored at an excitation wavelength of 360nm and an emission wavelength of 460 nm on a fluor...More data for this Ligand-Target Pair
Affinity DataIC50: 0.00900nMpH: 7.0 T: 2°CAssay Description:The substrate hydrolysis with or without inhibitor was monitored at an excitation wavelength of 360nm and an emission wavelength of 460 nm on a fluor...More data for this Ligand-Target Pair
Affinity DataIC50: 0.0100nMpH: 7.0 T: 2°CAssay Description:The substrate hydrolysis with or without inhibitor was monitored at an excitation wavelength of 360nm and an emission wavelength of 460 nm on a fluor...More data for this Ligand-Target Pair
Affinity DataIC50: 0.0110nMpH: 7.0 T: 2°CAssay Description:The substrate hydrolysis with or without inhibitor was monitored at an excitation wavelength of 360nm and an emission wavelength of 460 nm on a fluor...More data for this Ligand-Target Pair
Affinity DataIC50: 0.0110nMpH: 7.0 T: 2°CAssay Description:The substrate hydrolysis with or without inhibitor was monitored at an excitation wavelength of 360nm and an emission wavelength of 460 nm on a fluor...More data for this Ligand-Target Pair
Affinity DataIC50: 0.0130nMpH: 7.0 T: 2°CAssay Description:The substrate hydrolysis with or without inhibitor was monitored at an excitation wavelength of 360nm and an emission wavelength of 460 nm on a fluor...More data for this Ligand-Target Pair
Affinity DataIC50: 0.0220nMpH: 7.0 T: 2°CAssay Description:The substrate hydrolysis with or without inhibitor was monitored at an excitation wavelength of 360nm and an emission wavelength of 460 nm on a fluor...More data for this Ligand-Target Pair
Affinity DataIC50: 0.0250nMpH: 7.0 T: 2°CAssay Description:The substrate hydrolysis with or without inhibitor was monitored at an excitation wavelength of 360nm and an emission wavelength of 460 nm on a fluor...More data for this Ligand-Target Pair
Affinity DataIC50: 0.0310nMpH: 7.0 T: 2°CAssay Description:The substrate hydrolysis with or without inhibitor was monitored at an excitation wavelength of 360nm and an emission wavelength of 460 nm on a fluor...More data for this Ligand-Target Pair
Affinity DataIC50: 0.0470nMpH: 7.0 T: 2°CAssay Description:The substrate hydrolysis with or without inhibitor was monitored at an excitation wavelength of 360nm and an emission wavelength of 460 nm on a fluor...More data for this Ligand-Target Pair
Affinity DataIC50: 0.130nMAssay Description:The substrate hydrolysis with or without inhibitor was monitored at an excitation wavelength of 360nm and an emission wavelength of 460 nm on a fluor...More data for this Ligand-Target Pair
Affinity DataIC50: 0.170nMpH: 5.5 T: 2°CAssay Description:The substrate hydrolysis with or without inhibitor was monitored at an excitation wavelength of 360nm and an emission wavelength of 460 nm on a fluor...More data for this Ligand-Target Pair
Affinity DataIC50: 0.230nMAssay Description:The substrate hydrolysis with or without inhibitor was monitored at an excitation wavelength of 360nm and an emission wavelength of 460 nm on a fluor...More data for this Ligand-Target Pair
Affinity DataIC50: 0.300nMpH: 7.0 T: 2°CAssay Description:The substrate hydrolysis with or without inhibitor was monitored at an excitation wavelength of 360nm and an emission wavelength of 460 nm on a fluor...More data for this Ligand-Target Pair
Affinity DataIC50: 0.310nMpH: 5.5 T: 2°CAssay Description:The substrate hydrolysis with or without inhibitor was monitored at an excitation wavelength of 360nm and an emission wavelength of 460 nm on a fluor...More data for this Ligand-Target Pair
Affinity DataIC50: 0.330nMpH: 5.5 T: 2°CAssay Description:The substrate hydrolysis with or without inhibitor was monitored at an excitation wavelength of 360nm and an emission wavelength of 460 nm on a fluor...More data for this Ligand-Target Pair
Affinity DataIC50: 0.340nMpH: 5.5 T: 2°CAssay Description:The substrate hydrolysis with or without inhibitor was monitored at an excitation wavelength of 360nm and an emission wavelength of 460 nm on a fluor...More data for this Ligand-Target Pair
Affinity DataIC50: 0.450nMpH: 5.5 T: 2°CAssay Description:The substrate hydrolysis with or without inhibitor was monitored at an excitation wavelength of 360nm and an emission wavelength of 460 nm on a fluor...More data for this Ligand-Target Pair
Affinity DataIC50: 0.480nMAssay Description:The substrate hydrolysis with or without inhibitor was monitored at an excitation wavelength of 360nm and an emission wavelength of 460 nm on a fluor...More data for this Ligand-Target Pair
Affinity DataIC50: 0.510nMAssay Description:The substrate hydrolysis with or without inhibitor was monitored at an excitation wavelength of 360nm and an emission wavelength of 460 nm on a fluor...More data for this Ligand-Target Pair
Affinity DataIC50: 0.520nMpH: 5.5 T: 2°CAssay Description:The substrate hydrolysis with or without inhibitor was monitored at an excitation wavelength of 360nm and an emission wavelength of 460 nm on a fluor...More data for this Ligand-Target Pair
Affinity DataIC50: 0.580nMpH: 5.5 T: 2°CAssay Description:The substrate hydrolysis with or without inhibitor was monitored at an excitation wavelength of 360nm and an emission wavelength of 460 nm on a fluor...More data for this Ligand-Target Pair
Affinity DataIC50: 0.680nMpH: 5.5 T: 2°CAssay Description:The substrate hydrolysis with or without inhibitor was monitored at an excitation wavelength of 360nm and an emission wavelength of 460 nm on a fluor...More data for this Ligand-Target Pair
Affinity DataIC50: 0.700nMAssay Description:The substrate hydrolysis with or without inhibitor was monitored at an excitation wavelength of 360nm and an emission wavelength of 460 nm on a fluor...More data for this Ligand-Target Pair
Affinity DataIC50: 0.75nMpH: 7.0 T: 2°CAssay Description:The substrate hydrolysis with or without inhibitor was monitored at an excitation wavelength of 360nm and an emission wavelength of 460 nm on a fluor...More data for this Ligand-Target Pair
Affinity DataIC50: 0.820nMpH: 5.5 T: 2°CAssay Description:The substrate hydrolysis with or without inhibitor was monitored at an excitation wavelength of 360nm and an emission wavelength of 460 nm on a fluor...More data for this Ligand-Target Pair
Affinity DataIC50: 0.900nMAssay Description:The substrate hydrolysis with or without inhibitor was monitored at an excitation wavelength of 360nm and an emission wavelength of 460 nm on a fluor...More data for this Ligand-Target Pair
Affinity DataIC50: 0.900nMAssay Description:The substrate hydrolysis with or without inhibitor was monitored at an excitation wavelength of 360nm and an emission wavelength of 460 nm on a fluor...More data for this Ligand-Target Pair
TargetMAP kinase-activated protein kinase 2(Homo sapiens (Human))
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Affinity DataIC50: 1nMAssay Description:Inhibition of human MK2 using hsp27 peptide biotinyl-AYSRALSRQLSSGVSEIRCOOH as substrate after 45 mins by FRET assayMore data for this Ligand-Target Pair
TargetMitogen-activated protein kinase 9(Homo sapiens (Human))
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Novartis Institutes For Biomedical Research
Curated by ChEMBL
TargetGamma-aminobutyric acid receptor subunit alpha-1(Homo sapiens (Human))
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Affinity DataIC50: 1nMAssay Description:Displacement of [3H]flumazenil from GABAA receptor subunit alpha-1 expressed in CHO cellsMore data for this Ligand-Target Pair
TargetGamma-aminobutyric acid receptor subunit alpha-1(Homo sapiens (Human))
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Affinity DataIC50: 1nMAssay Description:Displacement of [3H]flumazenil from GABAA receptor subunit alpha-1 expressed in CHO cellsMore data for this Ligand-Target Pair
TargetTyrosine-protein kinase SYK(Homo sapiens (Human))
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Affinity DataIC50: 1nMAssay Description:Inhibition of Syk (unknown origin) using 5-Fluo-Ahx-GAPDYENLQELNKK-Amide as substrate after 60 mins by microfluidic mobility shift assayMore data for this Ligand-Target Pair
Affinity DataIC50: <1nMpH: 7.0 T: 2°CAssay Description:The substrate hydrolysis with or without inhibitor was monitored at an excitation wavelength of 360nm and an emission wavelength of 460 nm on a fluor...More data for this Ligand-Target Pair
TargetTyrosine-protein kinase SYK(Homo sapiens (Human))
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Affinity DataIC50: 1nMAssay Description:Inhibition of Syk (unknown origin) using 5-Fluo-Ahx-GAPDYENLQELNKK-Amide as substrate after 60 mins by microfluidic mobility shift assayMore data for this Ligand-Target Pair
Affinity DataIC50: <1nMpH: 7.0 T: 2°CAssay Description:The substrate hydrolysis with or without inhibitor was monitored at an excitation wavelength of 360nm and an emission wavelength of 460 nm on a fluor...More data for this Ligand-Target Pair
Affinity DataIC50: <1nMpH: 7.0 T: 2°CAssay Description:The substrate hydrolysis with or without inhibitor was monitored at an excitation wavelength of 360nm and an emission wavelength of 460 nm on a fluor...More data for this Ligand-Target Pair
Affinity DataIC50: 1.10nMpH: 5.5 T: 2°CAssay Description:The substrate hydrolysis with or without inhibitor was monitored at an excitation wavelength of 360nm and an emission wavelength of 460 nm on a fluor...More data for this Ligand-Target Pair
Affinity DataIC50: 1.20nMpH: 5.5 T: 2°CAssay Description:The substrate hydrolysis with or without inhibitor was monitored at an excitation wavelength of 360nm and an emission wavelength of 460 nm on a fluor...More data for this Ligand-Target Pair
Affinity DataIC50: 1.60nMAssay Description:The substrate hydrolysis with or without inhibitor was monitored at an excitation wavelength of 360nm and an emission wavelength of 460 nm on a fluor...More data for this Ligand-Target Pair
Affinity DataIC50: 1.70nMpH: 5.5 T: 2°CAssay Description:The substrate hydrolysis with or without inhibitor was monitored at an excitation wavelength of 360nm and an emission wavelength of 460 nm on a fluor...More data for this Ligand-Target Pair
Affinity DataIC50: 1.80nMAssay Description:The substrate hydrolysis with or without inhibitor was monitored at an excitation wavelength of 360nm and an emission wavelength of 460 nm on a fluor...More data for this Ligand-Target Pair
TargetMitogen-activated protein kinase 9(Homo sapiens (Human))
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Novartis Institutes For Biomedical Research
Curated by ChEMBL
TargetGamma-aminobutyric acid receptor subunit alpha-2(Homo sapiens (Human))
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Affinity DataIC50: 2nMAssay Description:Displacement of [3H]flumazenil from GABAA receptor subunit alpha-2 expressed in CHO cellsMore data for this Ligand-Target Pair
TargetHistone deacetylase 3(Homo sapiens (Human))
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Affinity DataIC50: 2.10nMAssay Description:Inhibition of HDAC3 (unknown origin) expressed in HEK293 cells using [3H]acetylated human histone H4 peptide as substrate by scintillation countingMore data for this Ligand-Target Pair
Affinity DataIC50: 2.30nMAssay Description:The substrate hydrolysis with or without inhibitor was monitored at an excitation wavelength of 360nm and an emission wavelength of 460 nm on a fluor...More data for this Ligand-Target Pair
TargetHistone deacetylase 1(Homo sapiens (Human))
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Affinity DataIC50: 2.5nMAssay Description:Inhibition of HDAC1 (unknown origin) expressed in HEK293 cells using [3H]acetylated human histone H4 peptide as substrate by scintillation countingMore data for this Ligand-Target Pair