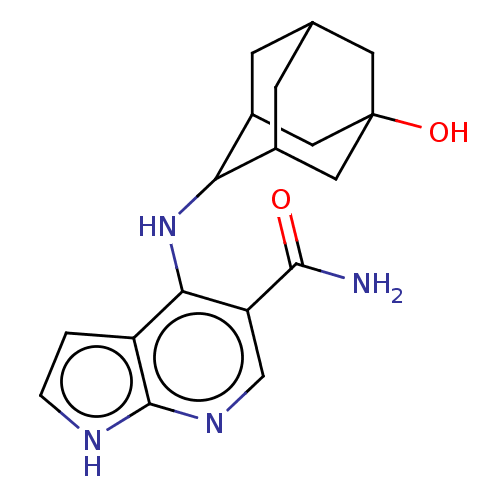
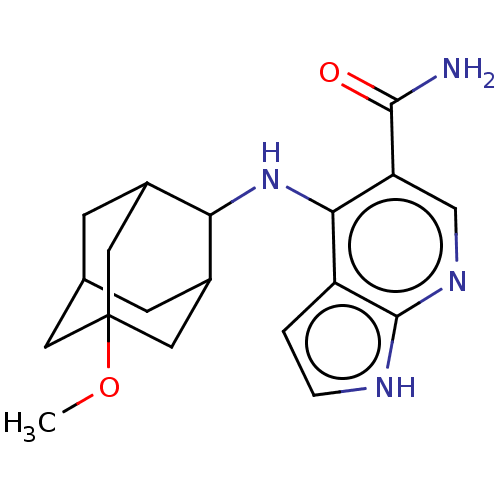
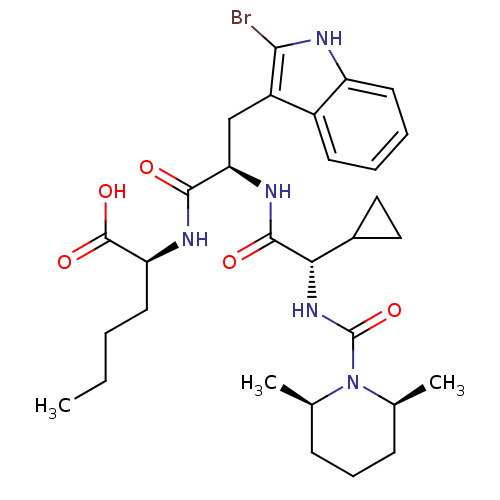
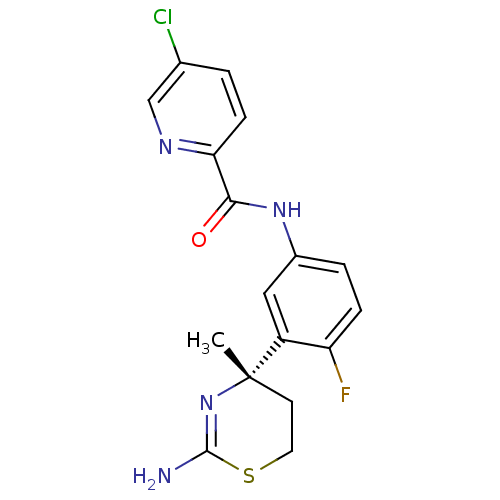
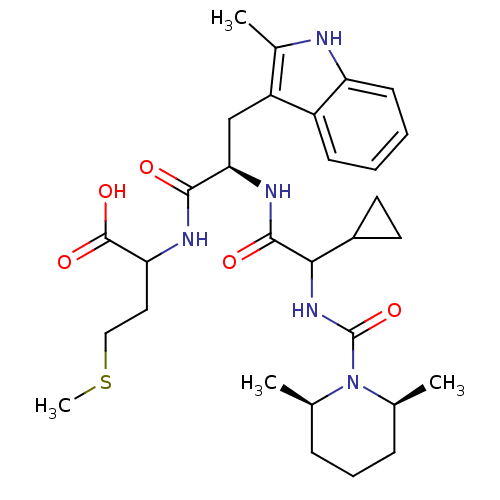
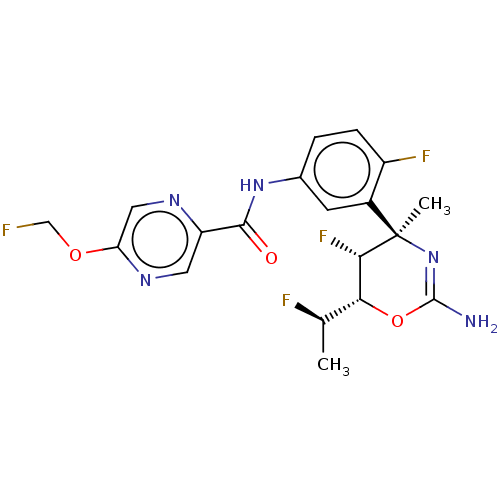
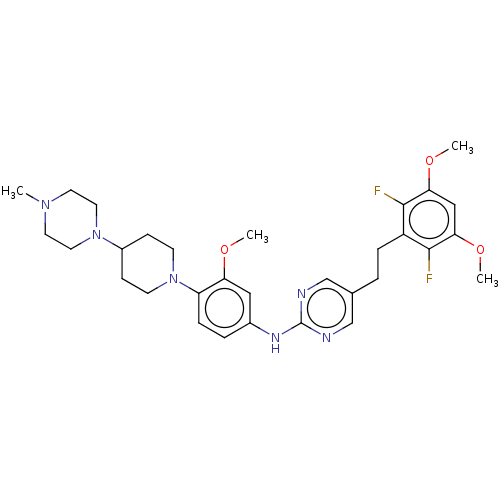
Affinity DataKi: 1.60nMAssay Description:Displacement of [3H]PDBu form PKCalphaMore data for this Ligand-Target Pair
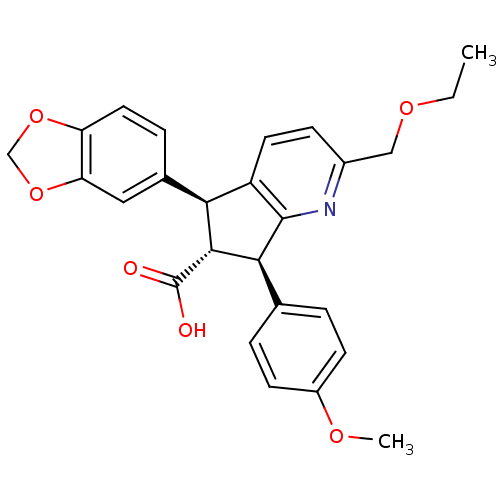
Affinity DataKi: 4.80nMAssay Description:Displacement of [3H]PDBu form PKCalphaMore data for this Ligand-Target Pair
TargetLysine-specific histone demethylase 1A(Homo sapiens (Human))
Nagoya City University
Curated by ChEMBL
Nagoya City University
Curated by ChEMBL
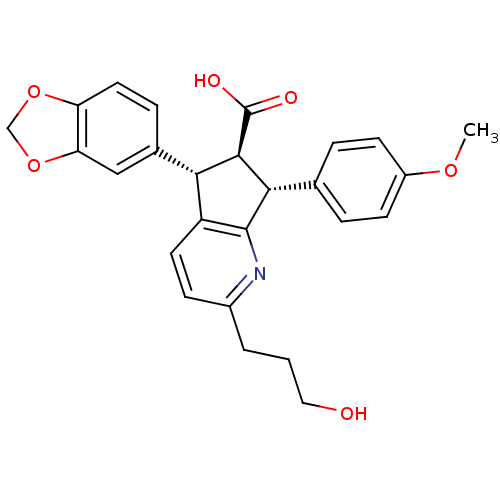
Affinity DataKi: 30nMAssay Description:Inhibition of human LSD1 (172 to 833 residues) assessed as reduction in H2O2 production using H3K4me2 (1 to 20 residues) peptide as substrate preincu...More data for this Ligand-Target Pair
TargetLysine-specific histone demethylase 1A(Homo sapiens (Human))
Nagoya City University
Curated by ChEMBL
Nagoya City University
Curated by ChEMBL
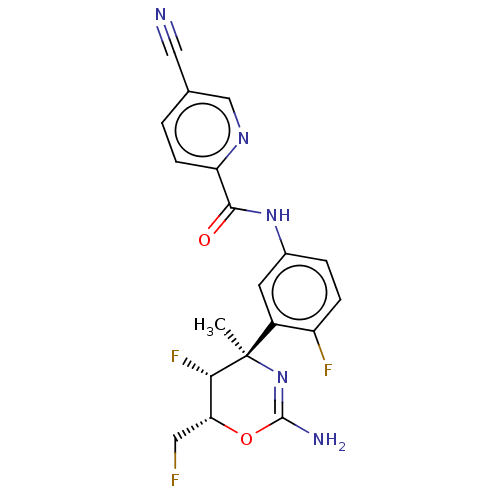
Affinity DataKi: 40nMAssay Description:Inhibition of human LSD1 assessed as reduction in H2O2 production using pLys4Met H3 peptide as substrate by peroxidase coupled UV-visible spectrophot...More data for this Ligand-Target Pair
TargetLysine-specific histone demethylase 1A(Homo sapiens (Human))
Nagoya City University
Curated by ChEMBL
Nagoya City University
Curated by ChEMBL
Affinity DataKi: 60nMAssay Description:Inhibition of human LSD1 (172 to 833 residues) assessed as reduction in H2O2 production using H3K4me2 (1 to 20 residues) peptide as substrate preincu...More data for this Ligand-Target Pair
TargetLysine-specific histone demethylase 1A(Homo sapiens (Human))
Nagoya City University
Curated by ChEMBL
Nagoya City University
Curated by ChEMBL
Affinity DataKi: 98nMAssay Description:Inhibition of human LSD1 (172 to 833 residues) assessed as reduction in H2O2 production using H3K4me2 (1 to 20 residues) peptide as substrate preincu...More data for this Ligand-Target Pair
Affinity DataKi: 118nMAssay Description:Displacement of [3H]PDBu form PKCalphaMore data for this Ligand-Target Pair
Affinity DataKi: 140nMAssay Description:Inhibition of recombinant human His-tagged LSD1 (171 to 836 residues)/GST-tagged CoREST (308 to 440 residues) complex using H3K4 peptide substrate by...More data for this Ligand-Target Pair
Affinity DataKi: 188nMAssay Description:Displacement of [3H]PDBu form PKCalphaMore data for this Ligand-Target Pair
TargetLysine-specific histone demethylase 1A(Homo sapiens (Human))
Nagoya City University
Curated by ChEMBL
Nagoya City University
Curated by ChEMBL
Affinity DataKi: 290nMAssay Description:Inhibition of human LSD1 (172 to 833 residues) assessed as reduction in H2O2 production using H3K4me2 (1 to 20 residues) peptide as substrate preincu...More data for this Ligand-Target Pair
TargetLysine-specific histone demethylase 1A(Homo sapiens (Human))
Nagoya City University
Curated by ChEMBL
Nagoya City University
Curated by ChEMBL
Affinity DataKi: 380nMAssay Description:Inhibition of human LSD1 (172 to 833 residues) assessed as reduction in H2O2 production using H3K4me2 (1 to 20 residues) peptide as substrate preincu...More data for this Ligand-Target Pair
TargetLysine-specific histone demethylase 1A(Homo sapiens (Human))
Nagoya City University
Curated by ChEMBL
Nagoya City University
Curated by ChEMBL
Affinity DataKi: 1.40E+3nMAssay Description:Inhibition of human LSD1 (172 to 833 residues) assessed as reduction in H2O2 production using H3K4me2 (1 to 20 residues) peptide as substrate preincu...More data for this Ligand-Target Pair
TargetLysine-specific histone demethylase 1A(Homo sapiens (Human))
Nagoya City University
Curated by ChEMBL
Nagoya City University
Curated by ChEMBL
Affinity DataKi: 1.60E+3nMAssay Description:Inhibition of human LSD1 assessed as reduction in H2O2 production using H3K4me2 (1 to 20 residues) peptide as substrate preincubated for 10 mins foll...More data for this Ligand-Target Pair
TargetLysine-specific histone demethylase 1A(Homo sapiens (Human))
Nagoya City University
Curated by ChEMBL
Nagoya City University
Curated by ChEMBL
Affinity DataKi: 1.90E+3nMAssay Description:Inhibition of human LSD1 assessed as reduction in H2O2 production using H3K4me2 (1 to 20 residues) peptide as substrate preincubated for 10 mins foll...More data for this Ligand-Target Pair
TargetLysine-specific histone demethylase 1A(Homo sapiens (Human))
Nagoya City University
Curated by ChEMBL
Nagoya City University
Curated by ChEMBL
Affinity DataKi: 6.30E+3nMAssay Description:Inhibition of human LSD1 (172 to 833 residues) assessed as reduction in H2O2 production using H3K4me2 (1 to 20 residues) peptide as substrate preincu...More data for this Ligand-Target Pair
TargetLysine-specific histone demethylase 1A(Homo sapiens (Human))
Nagoya City University
Curated by ChEMBL
Nagoya City University
Curated by ChEMBL
Affinity DataKi: 9.80E+3nMAssay Description:Inhibition of human LSD1 (172 to 833 residues) assessed as reduction in H2O2 production using H3K4me2 (1 to 20 residues) peptide as substrate preincu...More data for this Ligand-Target Pair
TargetLysine-specific histone demethylase 1A(Homo sapiens (Human))
Nagoya City University
Curated by ChEMBL
Nagoya City University
Curated by ChEMBL
Affinity DataKi: 3.70E+4nMAssay Description:Inhibition of human LSD1 assessed as reduction in H2O2 production using H3K4me2 (1 to 20 residues) peptide as substrate preincubated for 10 mins foll...More data for this Ligand-Target Pair
TargetLysine-specific histone demethylase 1A(Homo sapiens (Human))
Nagoya City University
Curated by ChEMBL
Nagoya City University
Curated by ChEMBL
Affinity DataKi: 4.40E+4nMAssay Description:Inhibition of human LSD1 assessed as reduction in H2O2 production using H3K4me2 (1 to 20 residues) peptide as substrate preincubated for 10 mins foll...More data for this Ligand-Target Pair
TargetLysine-specific histone demethylase 1A(Homo sapiens (Human))
Nagoya City University
Curated by ChEMBL
Nagoya City University
Curated by ChEMBL
Affinity DataKi: 1.70E+5nMAssay Description:Inhibition of human LSD1 (172 to 833 residues) assessed as reduction in H2O2 production using H3K4me2 (1 to 20 residues) peptide as substrate preincu...More data for this Ligand-Target Pair
TargetLysine-specific histone demethylase 1A(Homo sapiens (Human))
Nagoya City University
Curated by ChEMBL
Nagoya City University
Curated by ChEMBL
Affinity DataKi: 1.90E+5nMAssay Description:Inhibition of human LSD1 assessed as reduction in H2O2 production using H3K4me2 (1 to 20 residues) peptide as substrate preincubated for 10 mins foll...More data for this Ligand-Target Pair
TargetLysine-specific histone demethylase 1A(Homo sapiens (Human))
Nagoya City University
Curated by ChEMBL
Nagoya City University
Curated by ChEMBL
Affinity DataKi: >3.00E+5nMAssay Description:Inhibition of human LSD1 assessed as reduction in H2O2 production using H3K4me2 (1 to 20 residues) peptide as substrate preincubated for 10 mins foll...More data for this Ligand-Target Pair
TargetLysine-specific histone demethylase 1A(Homo sapiens (Human))
Nagoya City University
Curated by ChEMBL
Nagoya City University
Curated by ChEMBL
Affinity DataKi: >3.00E+5nMAssay Description:Inhibition of human LSD1 assessed as reduction in H2O2 production using H3K4me2 (1 to 20 residues) peptide as substrate preincubated for 10 mins foll...More data for this Ligand-Target Pair
TargetLysine-specific histone demethylase 1A(Homo sapiens (Human))
Nagoya City University
Curated by ChEMBL
Nagoya City University
Curated by ChEMBL
Affinity DataKi: >5.00E+5nMAssay Description:Inhibition of human LSD1 (172 to 833 residues) assessed as reduction in H2O2 production using H3K4me2 (1 to 20 residues) peptide as substrate preincu...More data for this Ligand-Target Pair
TargetLysine-specific histone demethylase 1A(Homo sapiens (Human))
Nagoya City University
Curated by ChEMBL
Nagoya City University
Curated by ChEMBL
Affinity DataKi: >5.00E+5nMAssay Description:Inhibition of human LSD1 (172 to 833 residues) assessed as reduction in H2O2 production using H3K4me2 (1 to 20 residues) peptide as substrate preincu...More data for this Ligand-Target Pair
TargetLysine-specific histone demethylase 1A(Homo sapiens (Human))
Nagoya City University
Curated by ChEMBL
Nagoya City University
Curated by ChEMBL
Affinity DataKi: >5.00E+5nMAssay Description:Inhibition of human LSD1 (172 to 833 residues) assessed as reduction in H2O2 production using H3K4me2 (1 to 20 residues) peptide as substrate preincu...More data for this Ligand-Target Pair
TargetLysine-specific histone demethylase 1A(Homo sapiens (Human))
Nagoya City University
Curated by ChEMBL
Nagoya City University
Curated by ChEMBL
Affinity DataKi: >5.00E+5nMAssay Description:Inhibition of human LSD1 (172 to 833 residues) assessed as reduction in H2O2 production using H3K4me2 (1 to 20 residues) peptide as substrate preincu...More data for this Ligand-Target Pair
Affinity DataIC50: 0.100nMAssay Description:Inhibitory activity of the compound against I-labeled ET-1 binding to Endothelin A receptorMore data for this Ligand-Target Pair
Affinity DataIC50: 0.120nMAssay Description:Inhibitory activity of the compound against I-labeled ET-1 binding to Endothelin A receptorMore data for this Ligand-Target Pair
Affinity DataIC50: 0.200nMAssay Description:Inhibitory activity of the compound against I-labeled ET-1 binding to Endothelin A receptorMore data for this Ligand-Target Pair
Affinity DataIC50: 0.210nMAssay Description:Inhibitory activity of the compound against I-labeled ET-1 binding to Endothelin A receptorMore data for this Ligand-Target Pair
Affinity DataIC50: 0.320nMAssay Description:Inhibitory activity of the compound against I-labeled ET-1 binding to Endothelin A receptorMore data for this Ligand-Target Pair
Affinity DataIC50: 0.370nMAssay Description:Inhibitory activity of the compound against I-labeled ET-1 binding to Endothelin A receptorMore data for this Ligand-Target Pair
Affinity DataIC50: 0.380nMAssay Description:Inhibition of BACE1 in human SH-SY5Y cells expressing human wild type amyloid precursor protein assessed as reduction in amyloidbeta40 production inc...More data for this Ligand-Target Pair
Affinity DataIC50: 0.430nMAssay Description:Inhibition of recombinant human N-terminal His-tagged JAK3 catalytic domain (795 to 1124 residues) expressed in baculovirus expression system using B...More data for this Ligand-Target Pair
Affinity DataIC50: 0.490nMAssay Description:Inhibition of recombinant human N-terminal GST-tagged JAK1 catalytic domain (850 to 1154 residues) expressed in baculovirus expression system using B...More data for this Ligand-Target Pair
Affinity DataIC50: 0.510nMAssay Description:Inhibitory activity of the compound against I-labeled ET-1 binding to Endothelin A receptorMore data for this Ligand-Target Pair
Affinity DataIC50: 0.550nMAssay Description:Inhibitory activity of the compound against I-labeled ET-1 binding to Endothelin A receptorMore data for this Ligand-Target Pair
Affinity DataIC50: 0.580nMAssay Description:Inhibition of BACE1 in human SH-SY5Y cells harboring wild-type human beta-APP assessed as reduction in secreted amyloid beta (1 to 40) after 24 hrs b...More data for this Ligand-Target Pair
Affinity DataIC50: 0.630nMAssay Description:Inhibition of recombinant human N-terminal His-tagged JAK3 catalytic domain (795 to 1124 residues) expressed in baculovirus expression system using B...More data for this Ligand-Target Pair
Affinity DataIC50: 0.710nMAssay Description:Inhibition of recombinant human N-terminal His-tagged JAK3 catalytic domain (795 to 1124 residues) expressed in baculovirus expression system using B...More data for this Ligand-Target Pair
Affinity DataIC50: 0.800nMAssay Description:Functional inhibition of ET-1 induced [Ca2+] increase in human Girardi heart (hGH) cells, which express Endothelin B receptorMore data for this Ligand-Target Pair
Affinity DataIC50: 0.810nMAssay Description:Binding affinity towards endothelin B receptor in porcine cerebellum membranesMore data for this Ligand-Target Pair
Affinity DataIC50: 0.810nMAssay Description:Inhibitory activity against 125 I-labeled ET-1 binding to Endothelin B receptor in porcine cerebellum membranesMore data for this Ligand-Target Pair
Affinity DataIC50: 0.840nMAssay Description:Inhibition of BACE1 (unknown origin) assessed as reduction in amyloid beta production by cell based assayMore data for this Ligand-Target Pair
Affinity DataIC50: 0.920nMAssay Description:Inhibitory activity of the compound against I-labeled ET-1 binding to Endothelin A receptorMore data for this Ligand-Target Pair
Affinity DataIC50: 1nMAssay Description:Binding affinity towards endothelin A receptor in porcine aortic smooth muscle membranes.More data for this Ligand-Target Pair
Affinity DataIC50: 1.10nMAssay Description:Inhibition of BACE1 in human SH-SY5Y cells harboring wild-type human beta-APP assessed as reduction in secreted amyloid beta (1 to 40) after 24 hrs b...More data for this Ligand-Target Pair
Affinity DataIC50: 1.10nMAssay Description:Binding affinity towards Endothelin B receptor in human girardi heart cell membranesMore data for this Ligand-Target Pair
Affinity DataIC50: 1.20nMAssay Description:Inhibition of human N-terminal GST-tagged FGFR3 (436 to end residues) expressed in baculovirus expression system using CSKtide as substrate incubated...More data for this Ligand-Target Pair
Affinity DataIC50: 1.20nMAssay Description:Inhibitory activity against 125 I-labeled ET-1 binding to Endothelin B receptor in porcine cerebellum membranesMore data for this Ligand-Target Pair

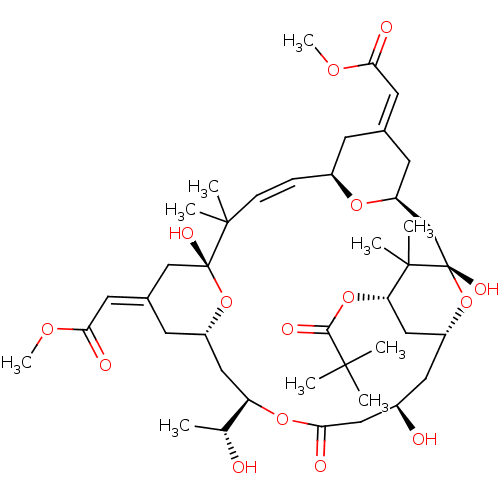

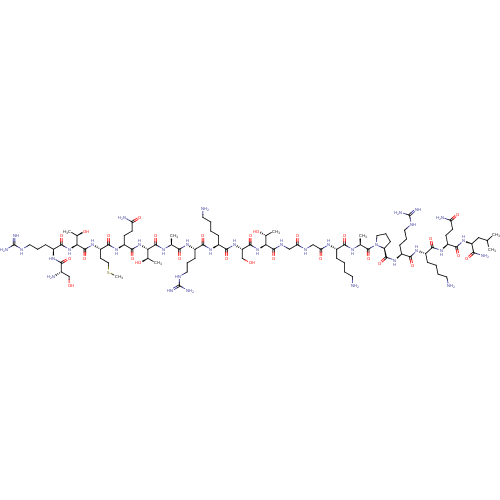

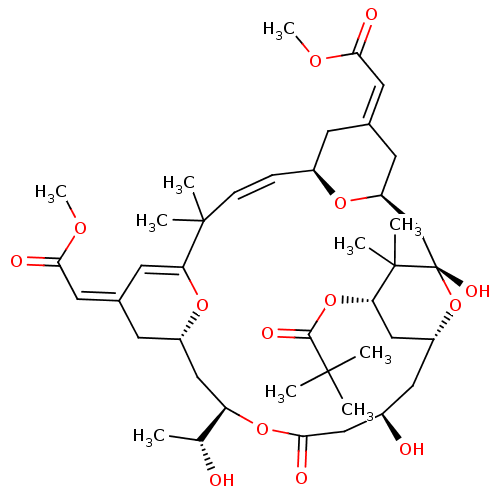
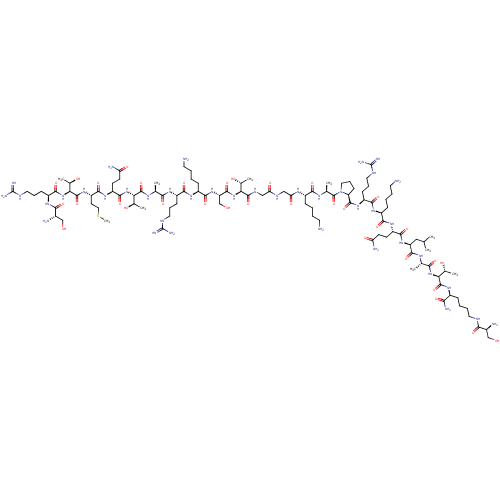

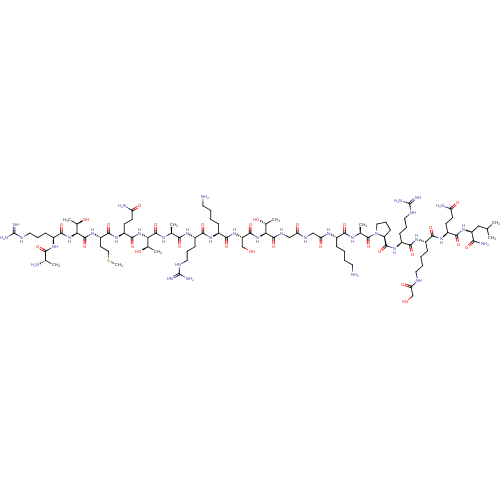

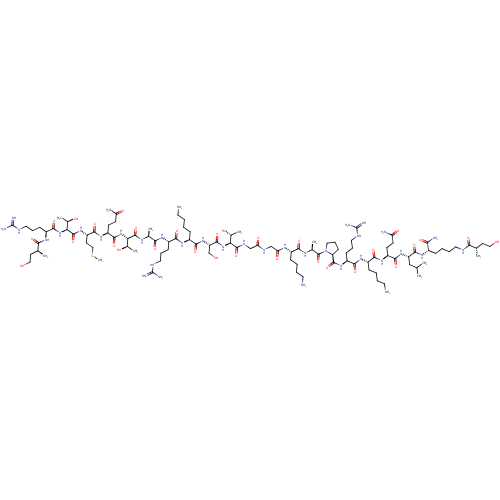

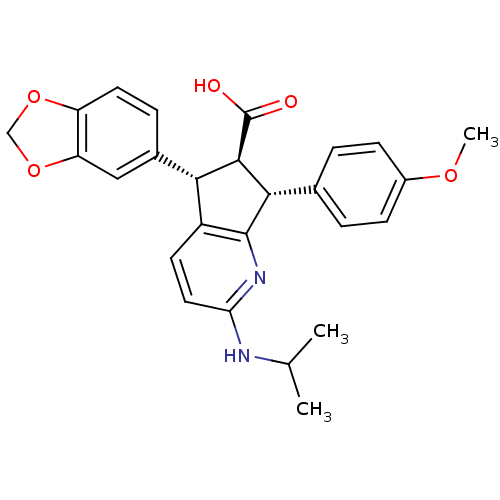

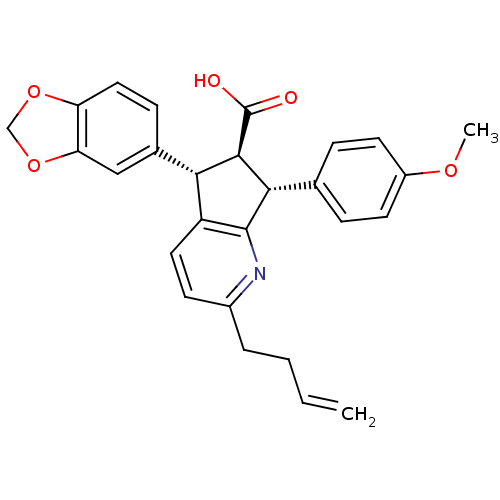

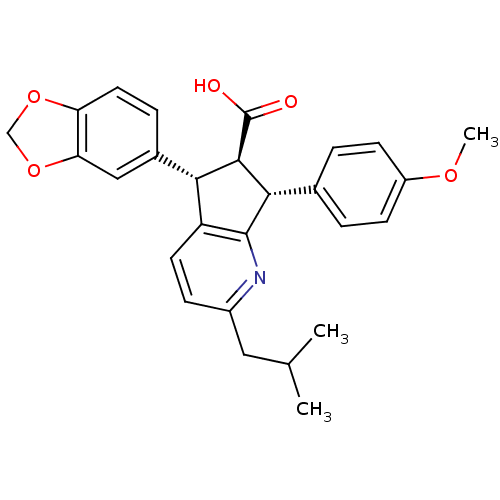
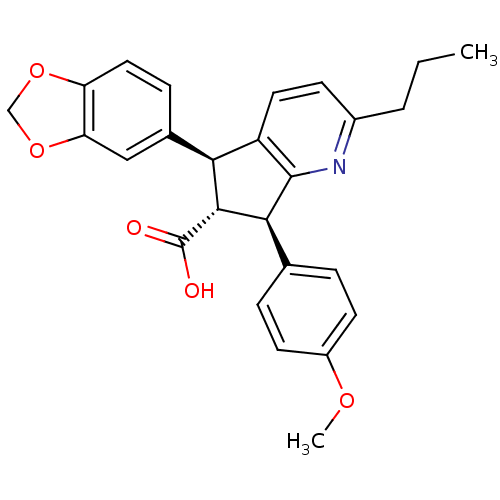
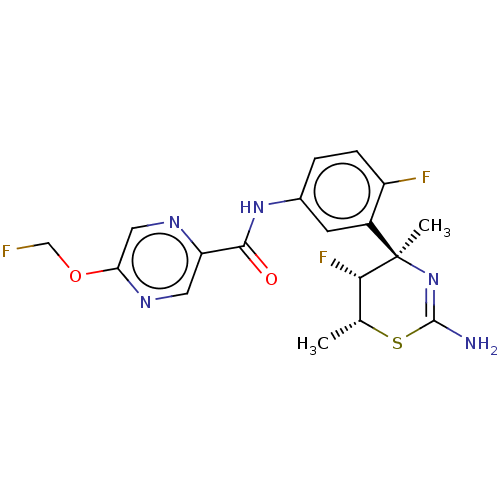
 3D Structure (crystal)
3D Structure (crystal)