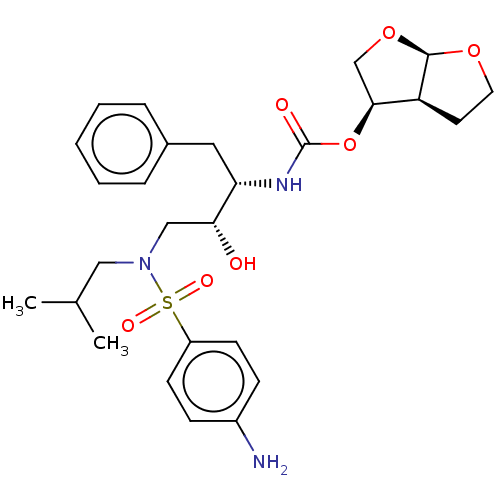
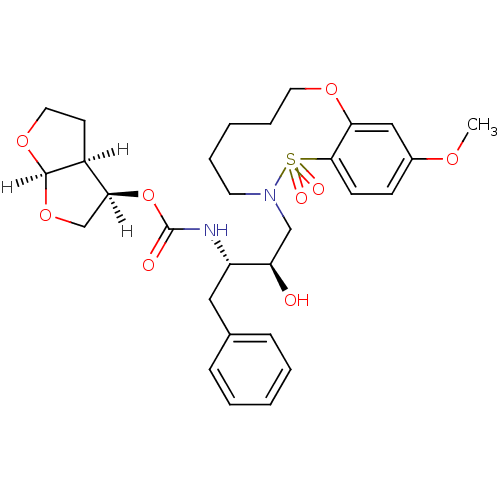
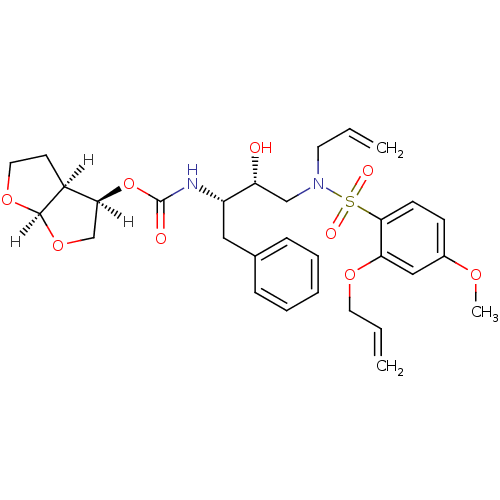
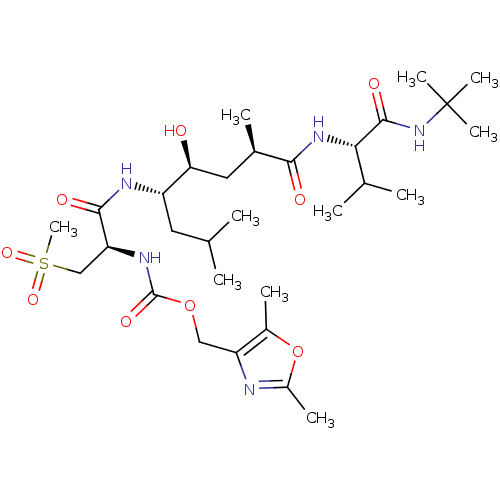
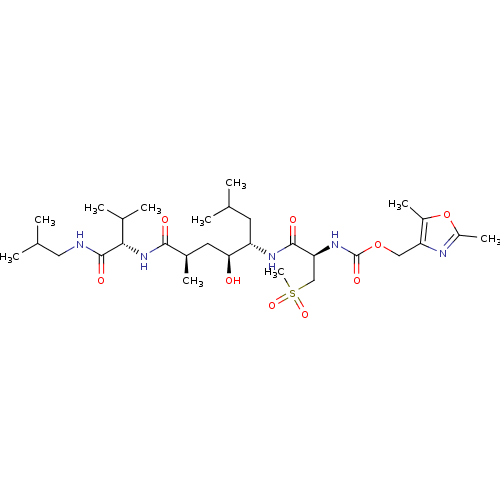
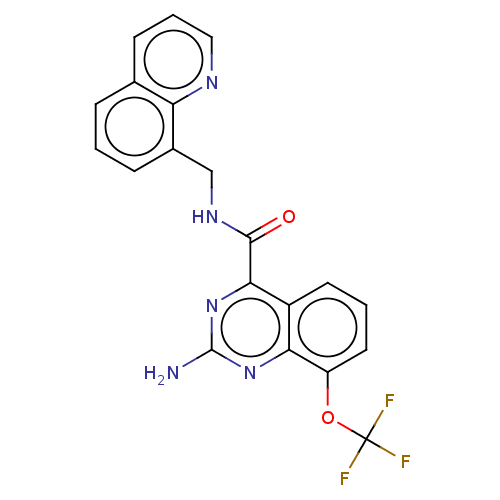
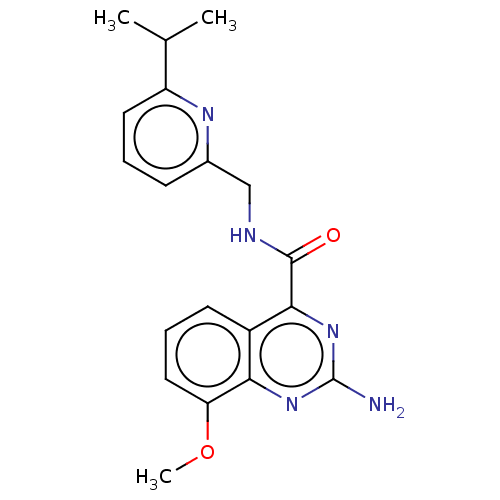
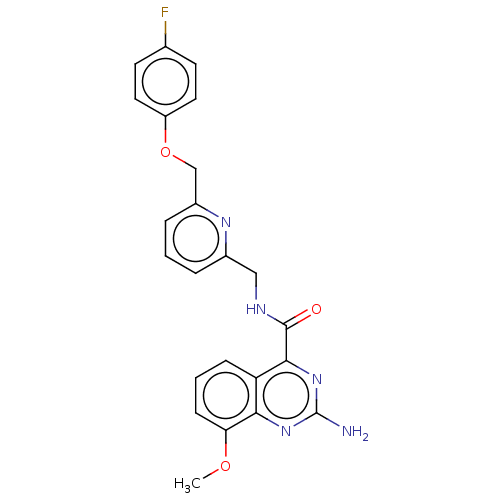
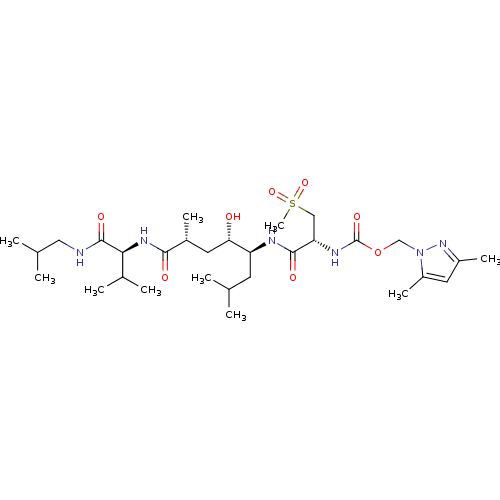
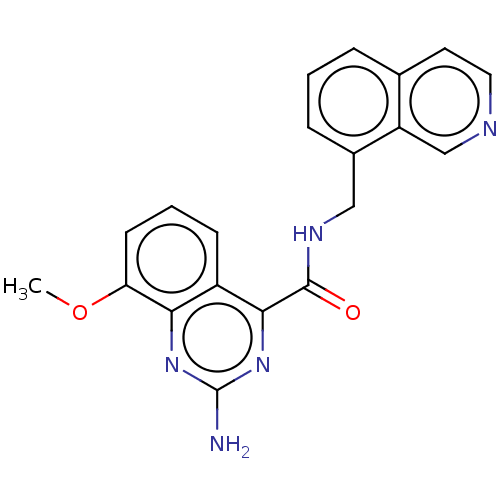
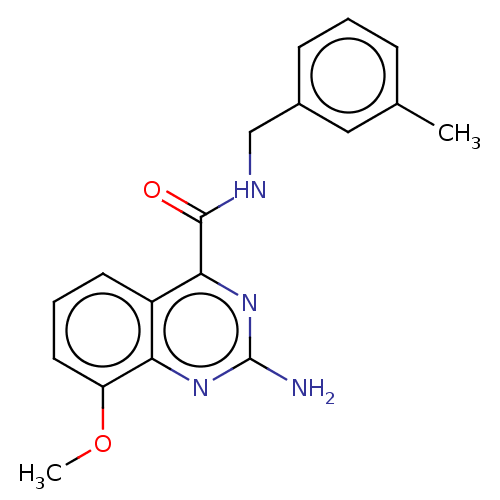
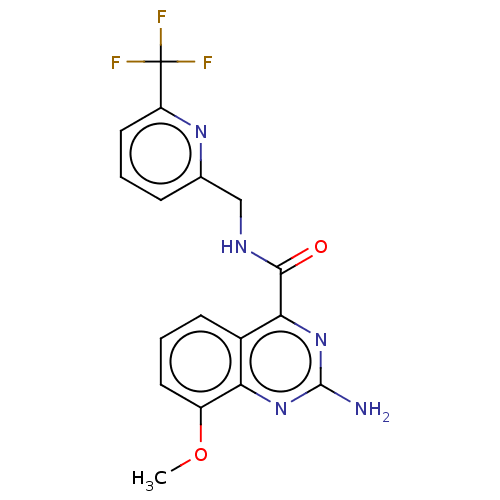
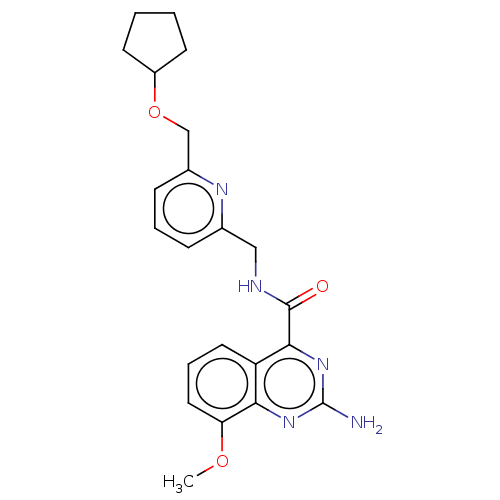
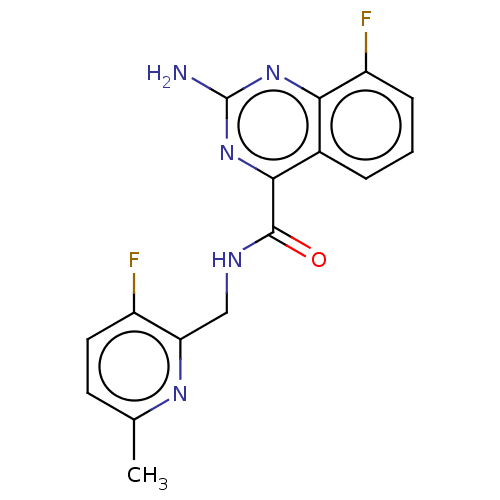
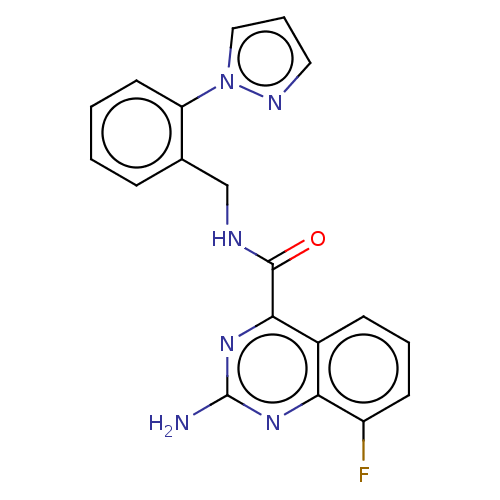
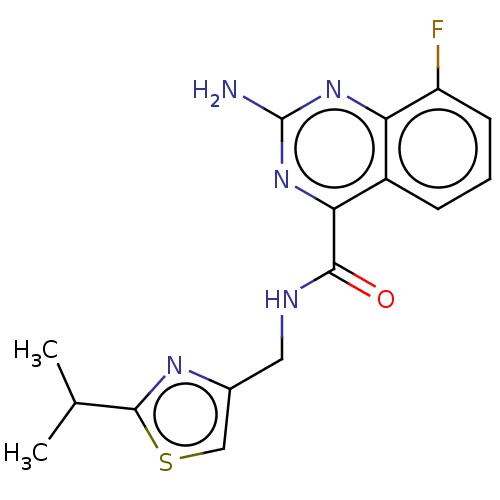
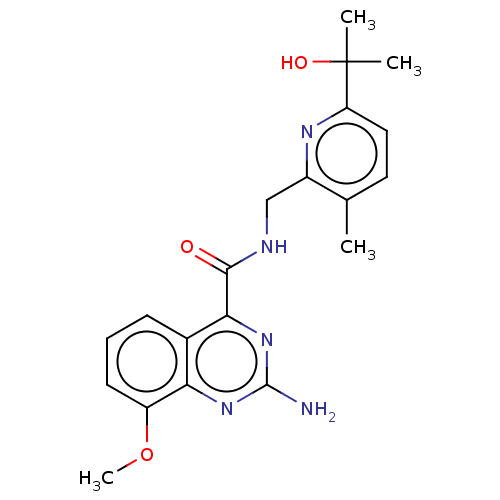
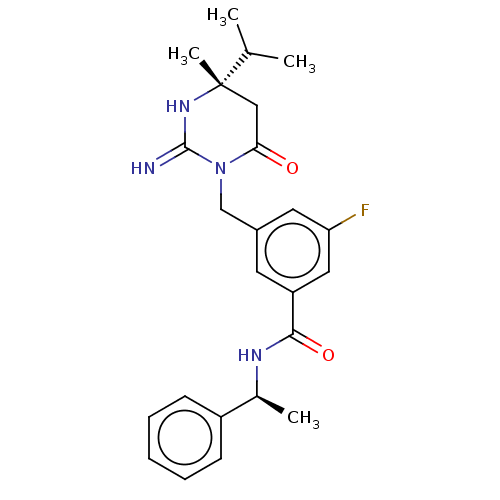
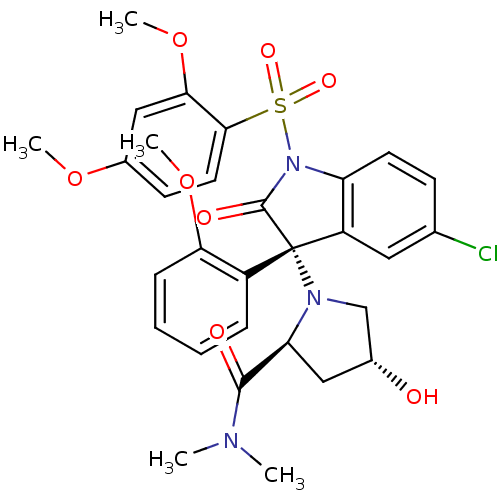
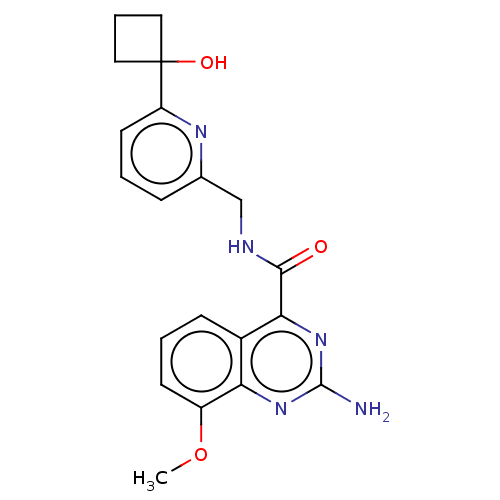
TargetDimer of Gag-Pol polyprotein [501-599,Q508K,L534I,L564I,C568A,C596A](Human immunodeficiency virus type 1)
Purdue University
Purdue University
Affinity DataKi: 0.0120nM ΔG°: -62.3kJ/mole EC50: 4.60nMpH: 6.4 T: 2°CAssay Description:The Ki values were determined by substrate cleavage assay using fluorogenic substrate, 2-(aminobenzoyl)-Thr-Ile-Nle-Phe(p-NO2)-Gln-Arg-NH2. A standar...More data for this Ligand-Target Pair
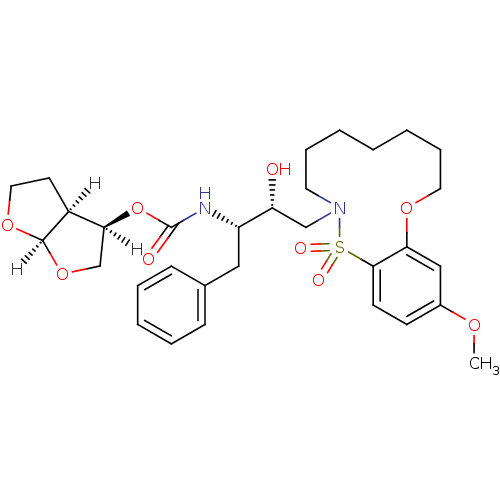
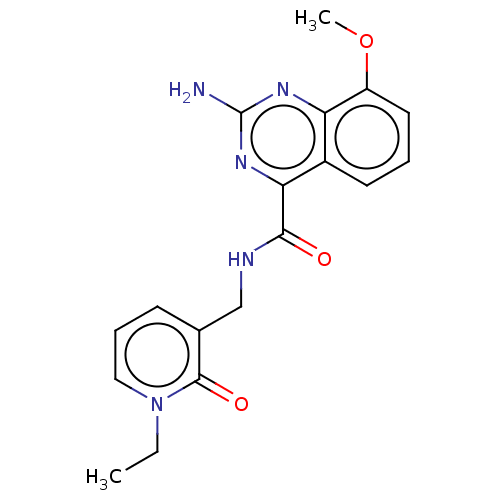
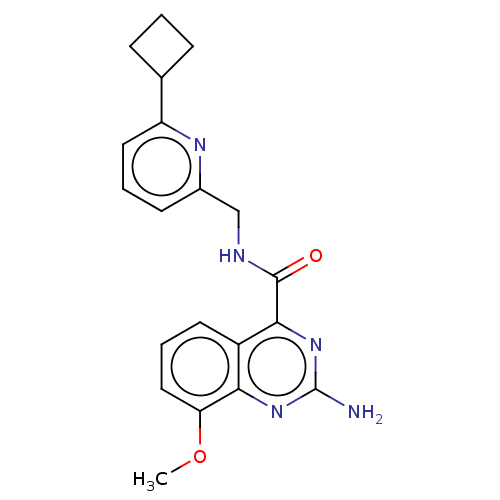
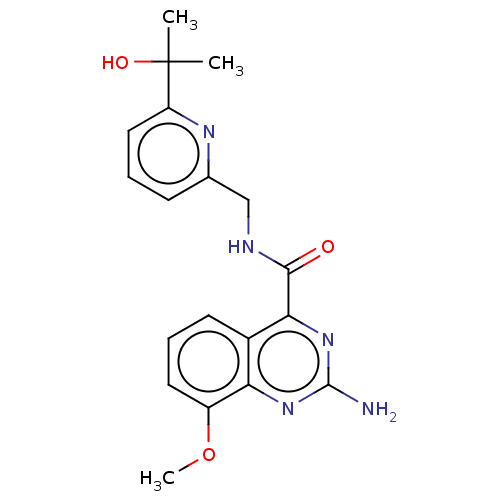
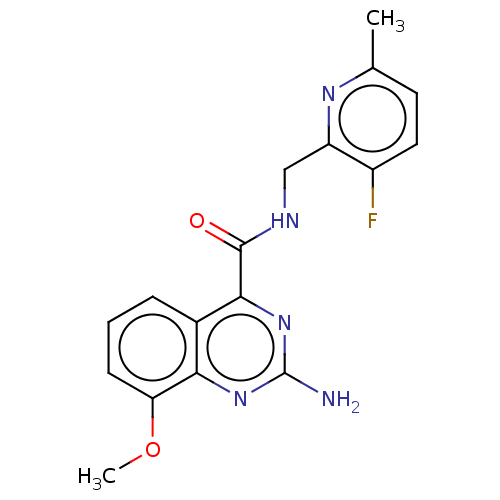
TargetDimer of Gag-Pol polyprotein [501-599,Q508K,L534I,L564I,C568A,C596A](Human immunodeficiency virus type 1)
Purdue University
Purdue University
Affinity DataKi: 0.0140nM ΔG°: -62.0kJ/mole EC50: 1.20nMpH: 6.4 T: 2°CAssay Description:The Ki values were determined by substrate cleavage assay using fluorogenic substrate, 2-(aminobenzoyl)-Thr-Ile-Nle-Phe(p-NO2)-Gln-Arg-NH2. A standar...More data for this Ligand-Target Pair
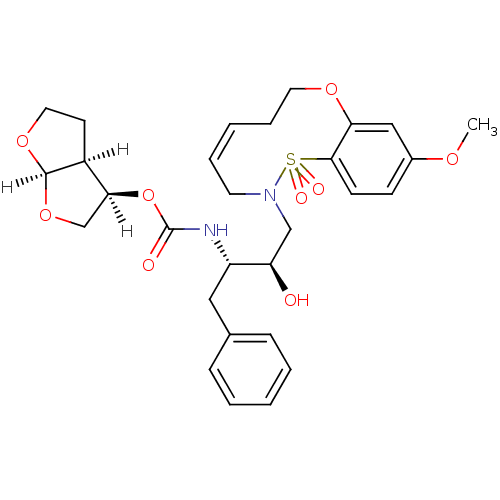
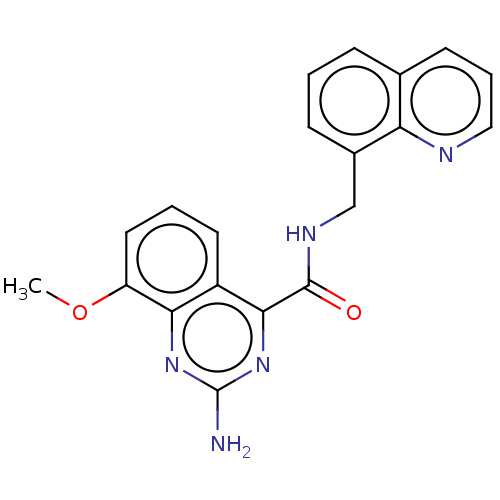
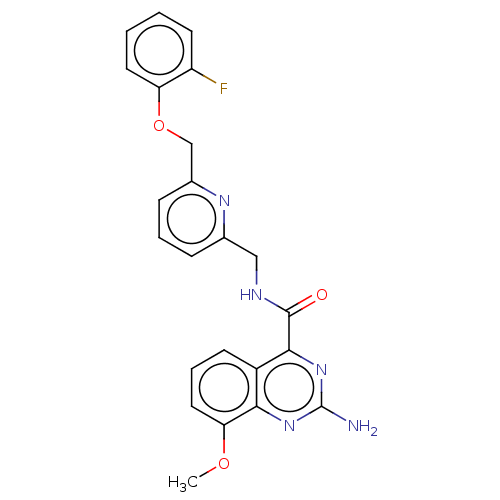
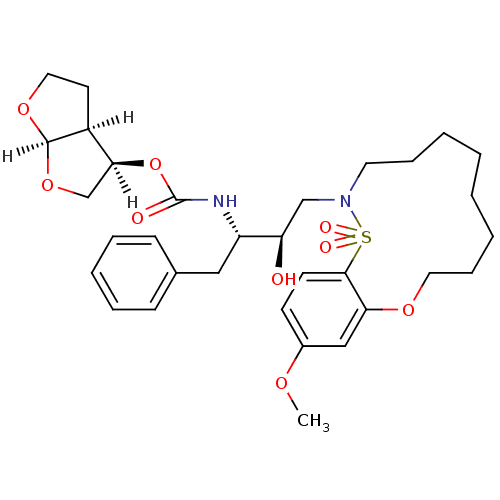
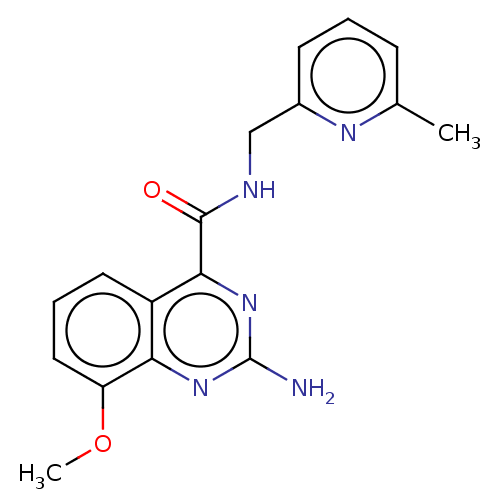
TargetDimer of Gag-Pol polyprotein [501-599,Q508K,L534I,L564I,C568A,C596A](Human immunodeficiency virus type 1)
Purdue University
Purdue University
Affinity DataKi: 0.0160nM ΔG°: -61.6kJ/mole EC50: 1.60nMpH: 6.4 T: 2°CAssay Description:The Ki values were determined by substrate cleavage assay using fluorogenic substrate, 2-(aminobenzoyl)-Thr-Ile-Nle-Phe(p-NO2)-Gln-Arg-NH2. A standar...More data for this Ligand-Target Pair
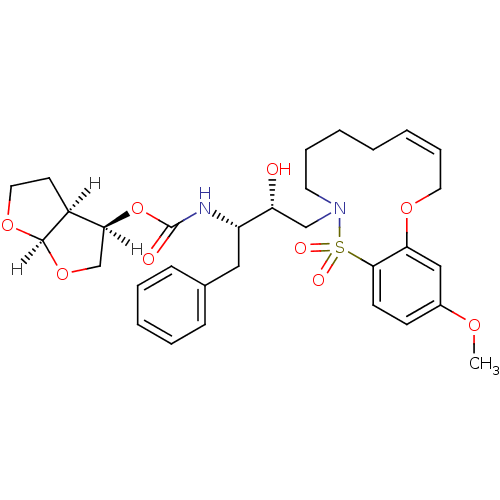
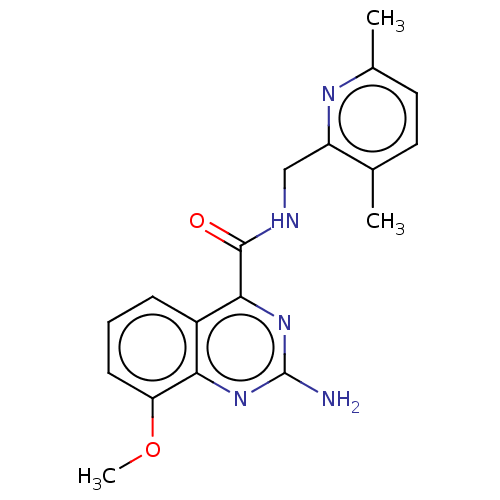
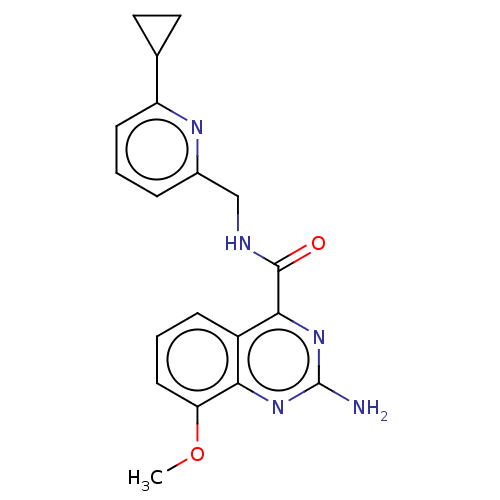
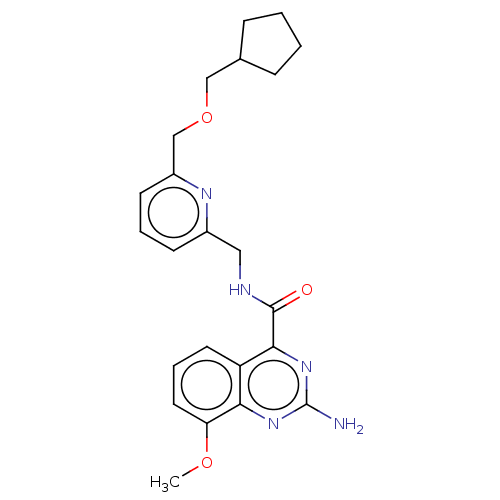
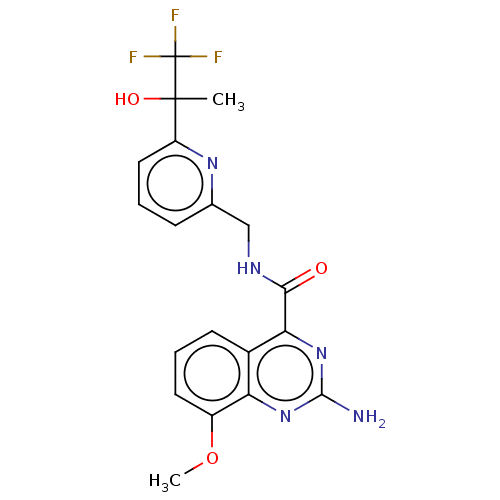
TargetDimer of Gag-Pol polyprotein [501-599,Q508K,L534I,L564I,C568A,C596A](Human immunodeficiency virus type 1)
Purdue University
Purdue University
Affinity DataKi: 0.0170nM EC50: 14nMAssay Description:The Ki values were determined by substrate cleavage assay using fluorogenic substrate, 2-(aminobenzoyl)-Thr-Ile-Nle-Phe(p-NO2)-Gln-Arg-NH2. A standar...More data for this Ligand-Target Pair
TargetDimer of Gag-Pol polyprotein [501-599,Q508K,L534I,L564I,C568A,C596A](Human immunodeficiency virus type 1)
Purdue University
Purdue University
Affinity DataKi: 0.0450nM ΔG°: -59.1kJ/mole EC50: 2nMpH: 6.4 T: 2°CAssay Description:The Ki values were determined by substrate cleavage assay using fluorogenic substrate, 2-(aminobenzoyl)-Thr-Ile-Nle-Phe(p-NO2)-Gln-Arg-NH2. A standar...More data for this Ligand-Target Pair
TargetDimer of Gag-Pol polyprotein [501-599,Q508K,L534I,L564I,C568A,C596A](Human immunodeficiency virus type 1)
Purdue University
Purdue University
Affinity DataKi: 0.0510nM ΔG°: -58.7kJ/mole EC50: 15nMpH: 6.4 T: 2°CAssay Description:The Ki values were determined by substrate cleavage assay using fluorogenic substrate, 2-(aminobenzoyl)-Thr-Ile-Nle-Phe(p-NO2)-Gln-Arg-NH2. A standar...More data for this Ligand-Target Pair
TargetDimer of Gag-Pol polyprotein [501-599,Q508K,L534I,L564I,C568A,C596A](Human immunodeficiency virus type 1)
Purdue University
Purdue University
Affinity DataKi: 0.0580nM ΔG°: -58.4kJ/mole EC50: 14nMpH: 6.4 T: 2°CAssay Description:The Ki values were determined by substrate cleavage assay using fluorogenic substrate, 2-(aminobenzoyl)-Thr-Ile-Nle-Phe(p-NO2)-Gln-Arg-NH2. A standar...More data for this Ligand-Target Pair
TargetDimer of Gag-Pol polyprotein [501-599,Q508K,L534I,L564I,C568A,C596A](Human immunodeficiency virus type 1)
Purdue University
Purdue University
Affinity DataKi: 0.0600nM ΔG°: -58.3kJ/mole EC50: 7nMpH: 6.4 T: 2°CAssay Description:The Ki values were determined by substrate cleavage assay using fluorogenic substrate, 2-(aminobenzoyl)-Thr-Ile-Nle-Phe(p-NO2)-Gln-Arg-NH2. A standar...More data for this Ligand-Target Pair
TargetDimer of Gag-Pol polyprotein [501-599,Q508K,L534I,L564I,C568A,C596A](Human immunodeficiency virus type 1)
Purdue University
Purdue University
Affinity DataKi: 0.0700nM EC50: 170nMAssay Description:The Ki values were determined by substrate cleavage assay using fluorogenic substrate, 2-(aminobenzoyl)-Thr-Ile-Nle-Phe(p-NO2)-Gln-Arg-NH2. A standar...More data for this Ligand-Target Pair
TargetDimer of Gag-Pol polyprotein [501-599,Q508K,L534I,L564I,C568A,C596A](Human immunodeficiency virus type 1)
Purdue University
Purdue University
Affinity DataKi: 0.0770nM ΔG°: -57.7kJ/mole EC50: 20nMpH: 6.4 T: 2°CAssay Description:The Ki values were determined by substrate cleavage assay using fluorogenic substrate, 2-(aminobenzoyl)-Thr-Ile-Nle-Phe(p-NO2)-Gln-Arg-NH2. A standar...More data for this Ligand-Target Pair
TargetDimer of Gag-Pol polyprotein [501-599,Q508K,L534I,L564I,C568A,C596A](Human immunodeficiency virus type 1)
Purdue University
Purdue University
Affinity DataKi: 0.0800nM ΔG°: -57.6kJ/mole EC50: 23nMpH: 6.4 T: 2°CAssay Description:The Ki values were determined by substrate cleavage assay using fluorogenic substrate, 2-(aminobenzoyl)-Thr-Ile-Nle-Phe(p-NO2)-Gln-Arg-NH2. A standar...More data for this Ligand-Target Pair
TargetDimer of Gag-Pol polyprotein [501-599,Q508K,L534I,L564I,C568A,C596A](Human immunodeficiency virus type 1)
Purdue University
Purdue University
Affinity DataKi: 0.0820nM ΔG°: -57.6kJ/mole EC50: 4nMpH: 6.4 T: 2°CAssay Description:The Ki values were determined by substrate cleavage assay using fluorogenic substrate, 2-(aminobenzoyl)-Thr-Ile-Nle-Phe(p-NO2)-Gln-Arg-NH2. A standar...More data for this Ligand-Target Pair
TargetDimer of Gag-Pol polyprotein [501-599,Q508K,L534I,L564I,C568A,C596A](Human immunodeficiency virus type 1)
Purdue University
Purdue University
Affinity DataKi: 0.0900nM ΔG°: -57.3kJ/mole EC50: 5.5nMpH: 6.4 T: 2°CAssay Description:The Ki values were determined by substrate cleavage assay using fluorogenic substrate, 2-(aminobenzoyl)-Thr-Ile-Nle-Phe(p-NO2)-Gln-Arg-NH2. A standar...More data for this Ligand-Target Pair
TargetDimer of Gag-Pol polyprotein [501-599,Q508K,L534I,L564I,C568A,C596A](Human immunodeficiency virus type 1)
Purdue University
Purdue University
Affinity DataKi: 0.100nM ΔG°: -57.1kJ/molepH: 6.4 T: 2°CAssay Description:The Ki values were determined by substrate cleavage assay using fluorogenic substrate, 2-(aminobenzoyl)-Thr-Ile-Nle-Phe(p-NO2)-Gln-Arg-NH2. A standar...More data for this Ligand-Target Pair
Affinity DataKi: 0.120nMAssay Description:Binding affinity to recombinant memapsin 2More data for this Ligand-Target Pair
Affinity DataKi: 0.120nM ΔG°: -58.9kJ/molepH: 4.5 T: 2°CAssay Description:Enzyme activities were assayed by monitoring the hydrolysis of substrate in the presence or absence of inhibitor compounds. The hydrolysis was record...More data for this Ligand-Target Pair
TargetDimer of Gag-Pol polyprotein [501-599,Q508K,L534I,L564I,C568A,C596A](Human immunodeficiency virus type 1)
Purdue University
Purdue University
Affinity DataKi: 0.150nM ΔG°: -56.1kJ/mole EC50: 95nMpH: 6.4 T: 2°CAssay Description:The Ki values were determined by substrate cleavage assay using fluorogenic substrate, 2-(aminobenzoyl)-Thr-Ile-Nle-Phe(p-NO2)-Gln-Arg-NH2. A standar...More data for this Ligand-Target Pair
TargetDimer of Gag-Pol polyprotein [501-599,Q508K,L534I,L564I,C568A,C596A](Human immunodeficiency virus type 1)
Purdue University
Purdue University
Affinity DataKi: 0.150nM EC50: 52nMAssay Description:The Ki values were determined by substrate cleavage assay using fluorogenic substrate, 2-(aminobenzoyl)-Thr-Ile-Nle-Phe(p-NO2)-Gln-Arg-NH2. A standar...More data for this Ligand-Target Pair
TargetDimer of Gag-Pol polyprotein [501-599,Q508K,L534I,L564I,C568A,C596A](Human immunodeficiency virus type 1)
Purdue University
Purdue University
Affinity DataKi: 0.160nM ΔG°: -55.9kJ/mole EC50: >1.00E+3nMpH: 6.4 T: 2°CAssay Description:The Ki values were determined by substrate cleavage assay using fluorogenic substrate, 2-(aminobenzoyl)-Thr-Ile-Nle-Phe(p-NO2)-Gln-Arg-NH2. A standar...More data for this Ligand-Target Pair
TargetDimer of Gag-Pol polyprotein [501-599,Q508K,L534I,L564I,C568A,C596A](Human immunodeficiency virus type 1)
Purdue University
Purdue University
Affinity DataKi: 0.180nM ΔG°: -55.6kJ/mole EC50: 6.60nMpH: 6.4 T: 2°CAssay Description:The Ki values were determined by substrate cleavage assay using fluorogenic substrate, 2-(aminobenzoyl)-Thr-Ile-Nle-Phe(p-NO2)-Gln-Arg-NH2. A standar...More data for this Ligand-Target Pair
Affinity DataKi: 0.200nMAssay Description:Binding affinities of compounds of the invention for the human A2a receptor were determined in a competition binding assay using Scintillation Proxim...More data for this Ligand-Target Pair
Affinity DataKi: 0.200nMAssay Description:Binding affinities of compounds of the invention for the human A2a receptor were determined in a competition binding assay using Scintillation Proxim...More data for this Ligand-Target Pair
Affinity DataKi: 0.200nMAssay Description:Binding affinities of compounds of the invention for the human A2a receptor were determined in a competition binding assay using Scintillation Proxim...More data for this Ligand-Target Pair
Affinity DataKi: 0.200nMAssay Description:Binding affinities of compounds of the invention for the human A2a receptor were determined in a competition binding assay using Scintillation Proxim...More data for this Ligand-Target Pair
Affinity DataKi: 0.200nMAssay Description:Binding affinities of compounds of the invention for the human A2a receptor were determined in a competition binding assay using Scintillation Proxim...More data for this Ligand-Target Pair
Affinity DataKi: 0.200nMAssay Description:Binding affinities of compounds of the invention for the human A2a receptor were determined in a competition binding assay using Scintillation Proxim...More data for this Ligand-Target Pair
Affinity DataKi: 0.200nMAssay Description:Binding affinities of compounds of the invention for the human A2a receptor were determined in a competition binding assay using Scintillation Proxim...More data for this Ligand-Target Pair
TargetDimer of Gag-Pol polyprotein [501-599,Q508K,L534I,L564I,C568A,C596A](Human immunodeficiency virus type 1)
Purdue University
Purdue University
Affinity DataKi: 0.240nM ΔG°: -54.9kJ/mole EC50: 360nMpH: 6.4 T: 2°CAssay Description:The Ki values were determined by substrate cleavage assay using fluorogenic substrate, 2-(aminobenzoyl)-Thr-Ile-Nle-Phe(p-NO2)-Gln-Arg-NH2. A standar...More data for this Ligand-Target Pair
Affinity DataKi: 0.300nMAssay Description:Binding affinities of compounds of the invention for the human A2a receptor were determined in a competition binding assay using Scintillation Proxim...More data for this Ligand-Target Pair
Affinity DataKi: 0.300nMAssay Description:Binding affinities of compounds of the invention for the human A2a receptor were determined in a competition binding assay using Scintillation Proxim...More data for this Ligand-Target Pair
Affinity DataKi: 0.300nM ΔG°: -56.5kJ/molepH: 4.5 T: 2°CAssay Description:Enzyme activities were assayed by monitoring the hydrolysis of substrate in the presence or absence of inhibitor compounds. The hydrolysis was record...More data for this Ligand-Target Pair
Affinity DataKi: 0.300nMAssay Description:Binding affinities of compounds of the invention for the human A2a receptor were determined in a competition binding assay using Scintillation Proxim...More data for this Ligand-Target Pair
TargetDimer of Gag-Pol polyprotein [501-599,Q508K,L534I,L564I,C568A,C596A](Human immunodeficiency virus type 1)
Purdue University
Purdue University
Affinity DataKi: 0.310nM EC50: 9nMAssay Description:The Ki values were determined by substrate cleavage assay using fluorogenic substrate, 2-(aminobenzoyl)-Thr-Ile-Nle-Phe(p-NO2)-Gln-Arg-NH2. A standar...More data for this Ligand-Target Pair
TargetDimer of Gag-Pol polyprotein [501-599,Q508K,L534I,L564I,C568A,C596A](Human immunodeficiency virus type 1)
Purdue University
Purdue University
Affinity DataKi: 0.380nM ΔG°: -53.8kJ/mole EC50: 120nMpH: 6.4 T: 2°CAssay Description:The Ki values were determined by substrate cleavage assay using fluorogenic substrate, 2-(aminobenzoyl)-Thr-Ile-Nle-Phe(p-NO2)-Gln-Arg-NH2. A standar...More data for this Ligand-Target Pair
Affinity DataKi: 0.400nMAssay Description:Binding affinities of compounds of the invention for the human A2a receptor were determined in a competition binding assay using Scintillation Proxim...More data for this Ligand-Target Pair
Affinity DataKi: 0.400nMAssay Description:Binding affinities of compounds of the invention for the human A2a receptor were determined in a competition binding assay using Scintillation Proxim...More data for this Ligand-Target Pair
Affinity DataKi: 0.400nMAssay Description:Binding affinities of compounds of the invention for the human A2a receptor were determined in a competition binding assay using Scintillation Proxim...More data for this Ligand-Target Pair
TargetDimer of Gag-Pol polyprotein [501-599,Q508K,L534I,L564I,C568A,C596A](Human immunodeficiency virus type 1)
Purdue University
Purdue University
Affinity DataKi: 0.470nM ΔG°: -53.2kJ/mole EC50: 22nMpH: 6.4 T: 2°CAssay Description:The Ki values were determined by substrate cleavage assay using fluorogenic substrate, 2-(aminobenzoyl)-Thr-Ile-Nle-Phe(p-NO2)-Gln-Arg-NH2. A standar...More data for this Ligand-Target Pair
Affinity DataKi: 0.5nMAssay Description:Binding affinities of compounds of the invention for the human A2a receptor were determined in a competition binding assay using Scintillation Proxim...More data for this Ligand-Target Pair
Affinity DataKi: 0.5nMAssay Description:Binding affinities of compounds of the invention for the human A2a receptor were determined in a competition binding assay using Scintillation Proxim...More data for this Ligand-Target Pair
Affinity DataKi: 0.5nMAssay Description:Binding affinities of compounds of the invention for the human A2a receptor were determined in a competition binding assay using Scintillation Proxim...More data for this Ligand-Target Pair
Affinity DataKi: 0.5nMAssay Description:Binding affinities of compounds of the invention for the human A2a receptor were determined in a competition binding assay using Scintillation Proxim...More data for this Ligand-Target Pair
Affinity DataKi: 0.5nMAssay Description:Binding affinities of compounds of the invention for the human A2a receptor were determined in a competition binding assay using Scintillation Proxim...More data for this Ligand-Target Pair
Affinity DataKi: 0.5nMAssay Description:Binding affinities of compounds of the invention for the human A2a receptor were determined in a competition binding assay using Scintillation Proxim...More data for this Ligand-Target Pair
Affinity DataKi: 0.5nMAssay Description:Binding affinities of compounds of the invention for the human A2a receptor were determined in a competition binding assay using Scintillation Proxim...More data for this Ligand-Target Pair
Affinity DataKi: 0.600nMAssay Description:Binding affinities of compounds of the invention for the human A2a receptor were determined in a competition binding assay using Scintillation Proxim...More data for this Ligand-Target Pair
Affinity DataKi: 0.600nMAssay Description:Binding affinities of compounds of the invention for the human A2a receptor were determined in a competition binding assay using Scintillation Proxim...More data for this Ligand-Target Pair
Affinity DataKi: 0.600nMAssay Description:Inhibition of renin (unknown origin)More data for this Ligand-Target Pair
TargetVasopressin V1b receptor(Homo sapiens (Human))
Schering-Plough Research Institute
Curated by ChEMBL
Schering-Plough Research Institute
Curated by ChEMBL
Affinity DataKi: 0.600nMAssay Description:Displacement of [3H]Arg8-vasopressin from human vasopressin V1b receptor expressed in CHO-K1 cells by Packard Topcount scintillation counterMore data for this Ligand-Target Pair
Affinity DataKi: 0.600nMAssay Description:Binding affinities of compounds of the invention for the human A2a receptor were determined in a competition binding assay using Scintillation Proxim...More data for this Ligand-Target Pair

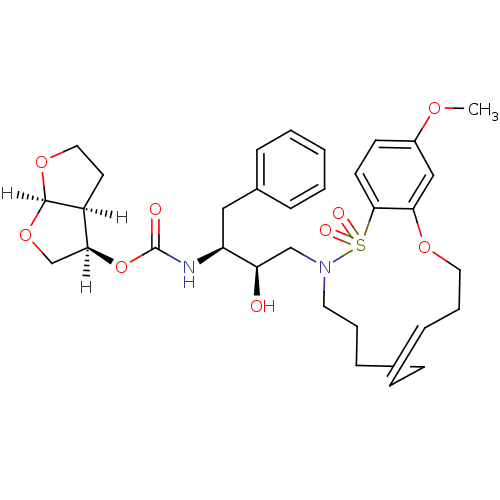
 3D Structure (crystal)
3D Structure (crystal)