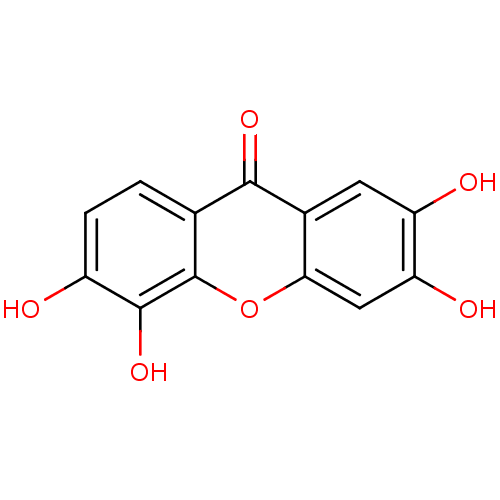
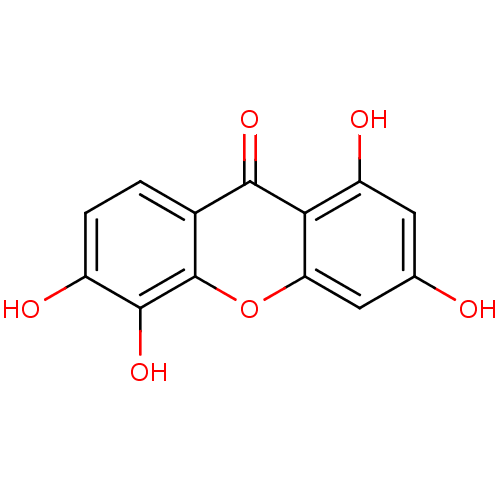
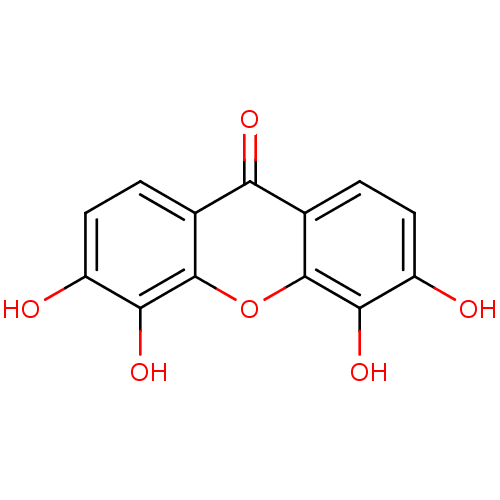
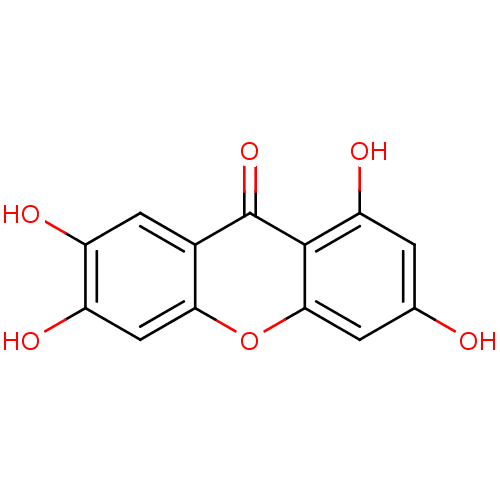
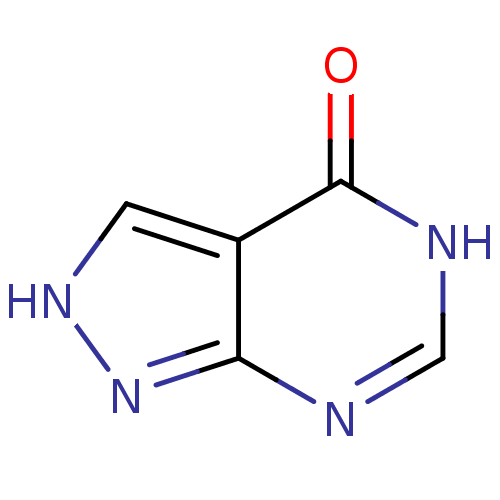
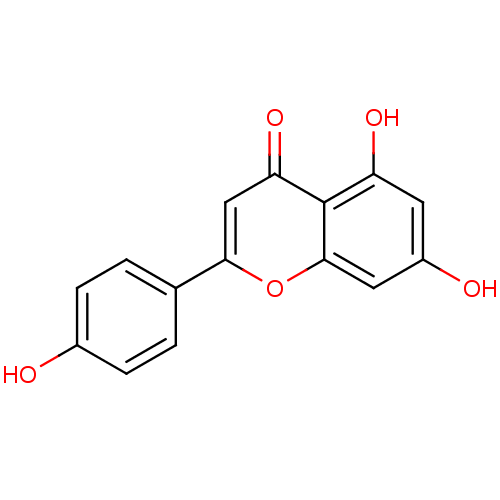
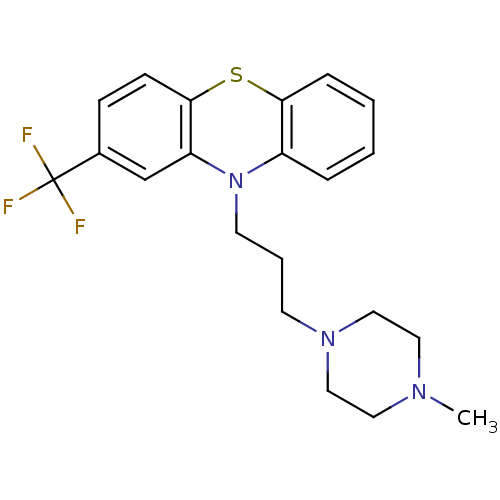
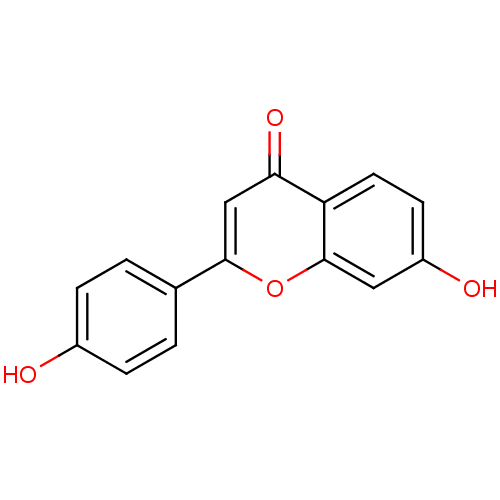
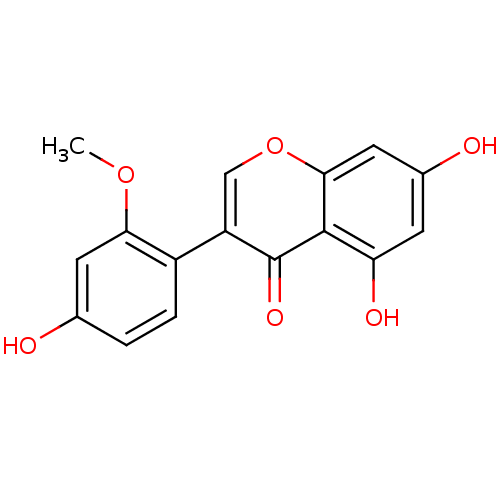
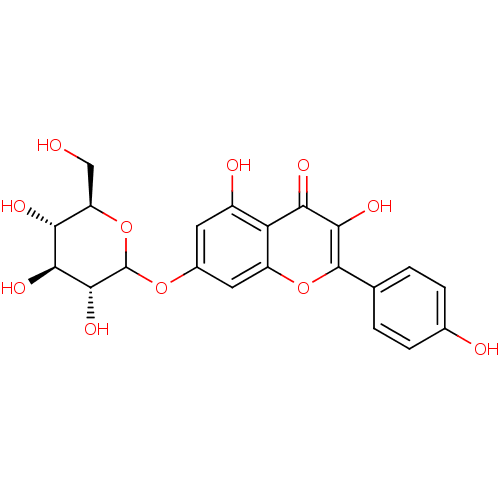
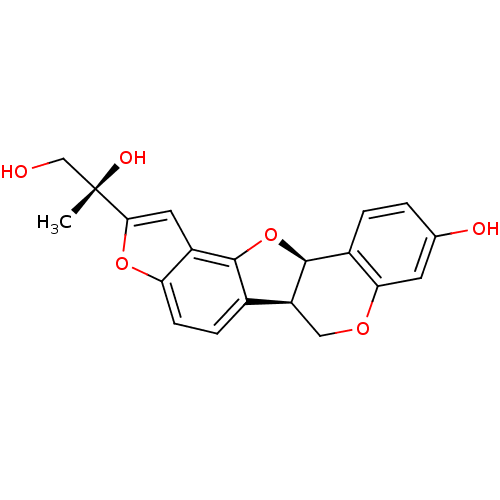
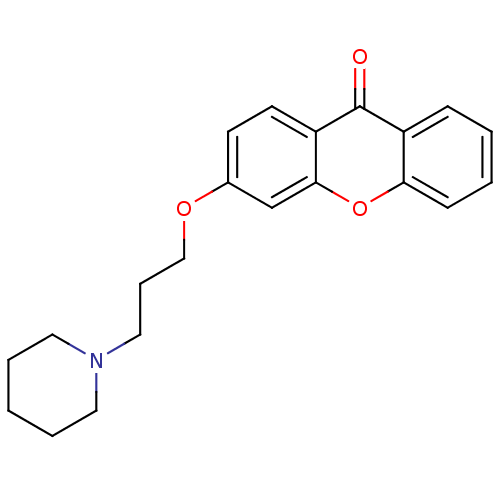
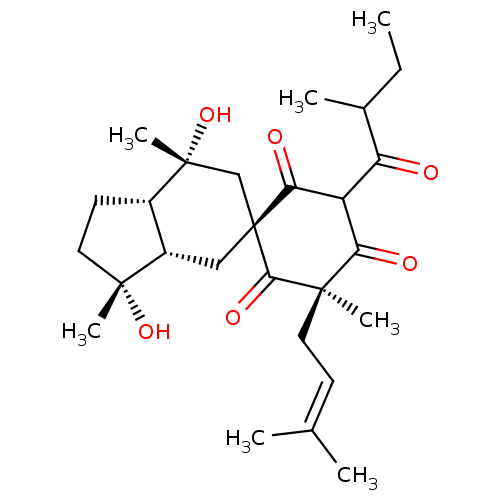
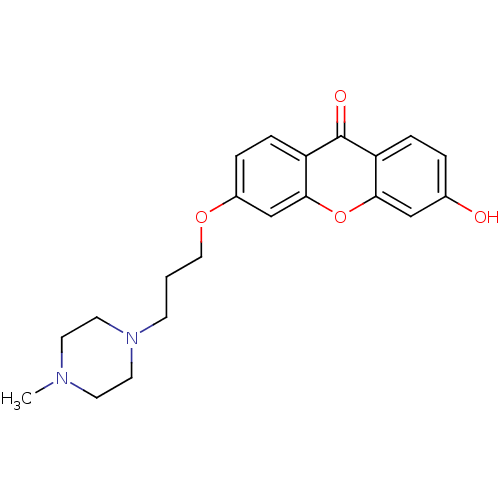
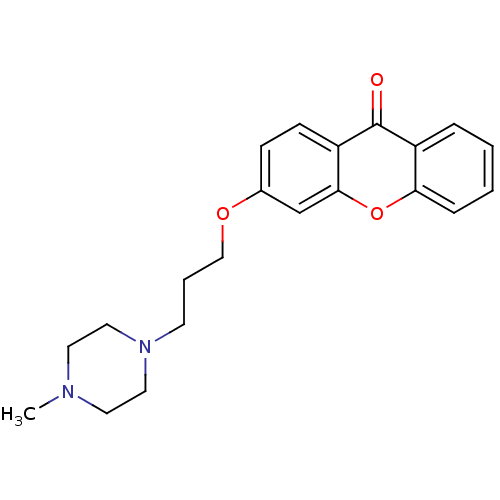
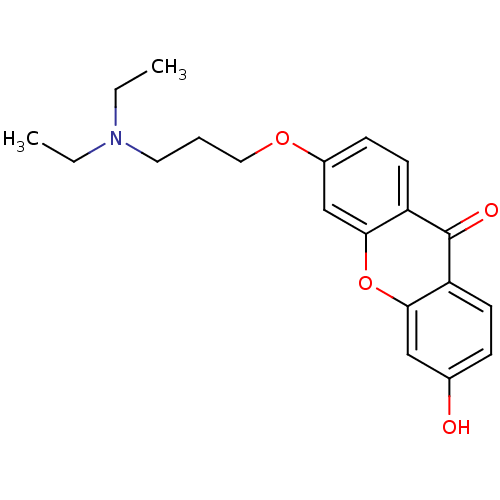
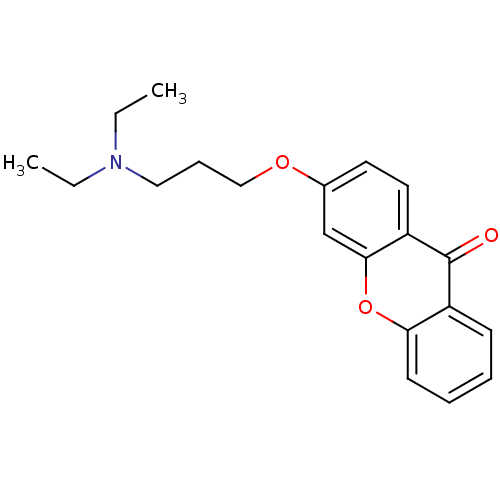
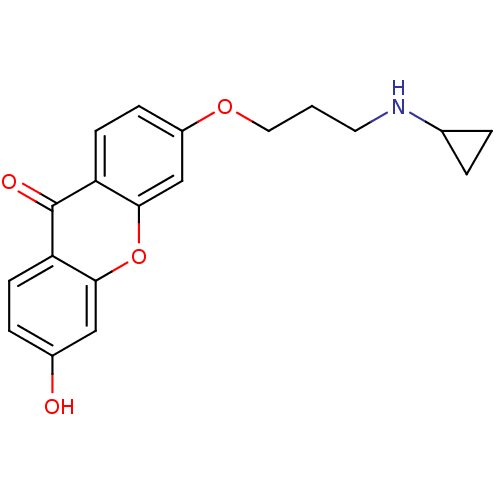
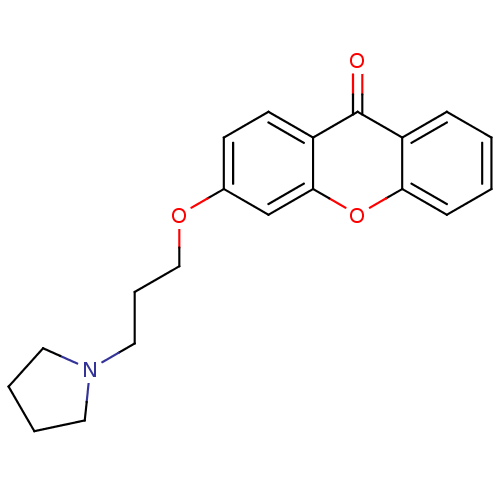
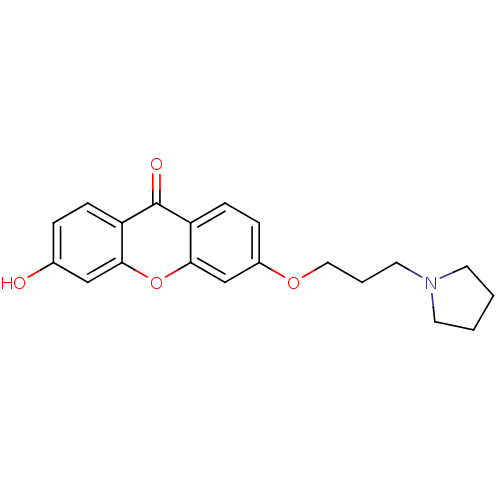
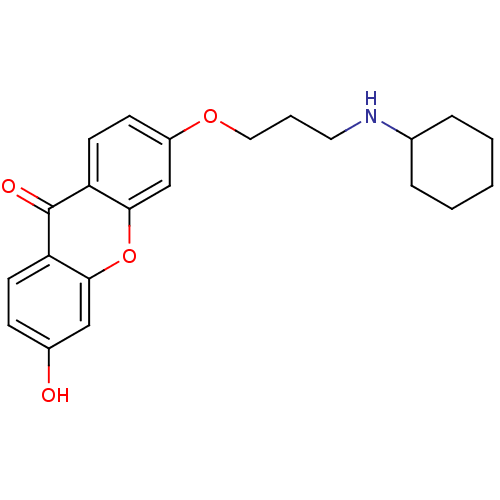
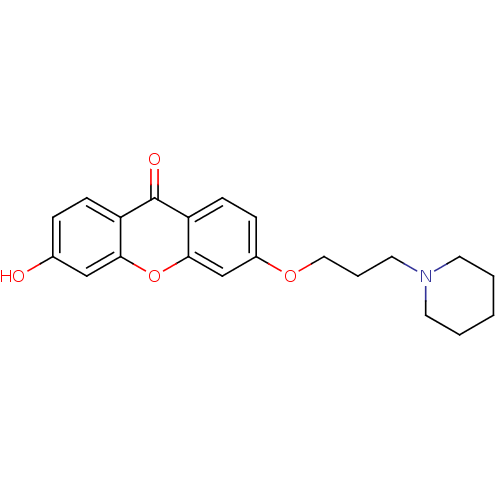
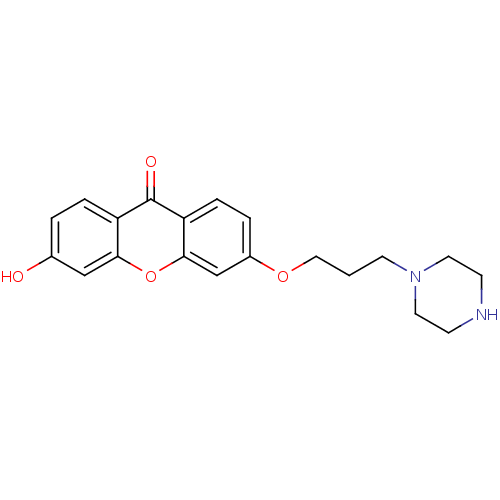
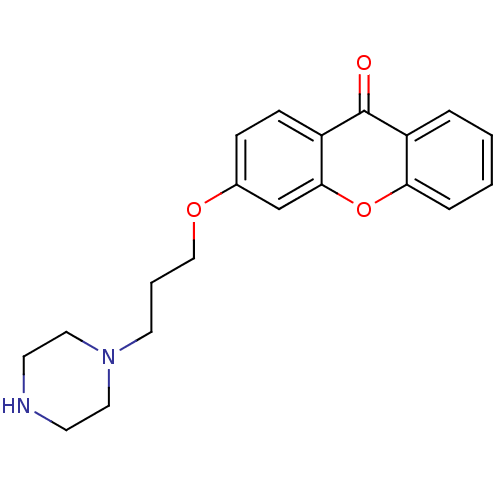
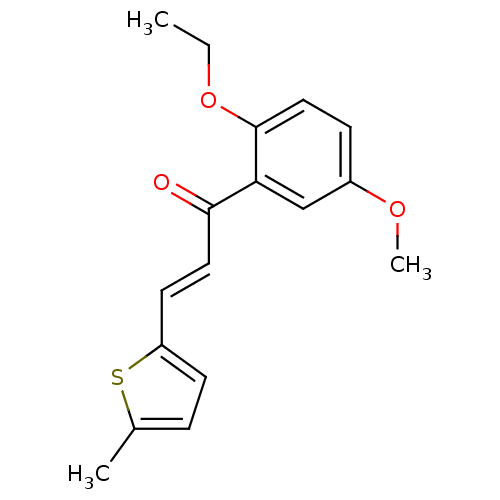
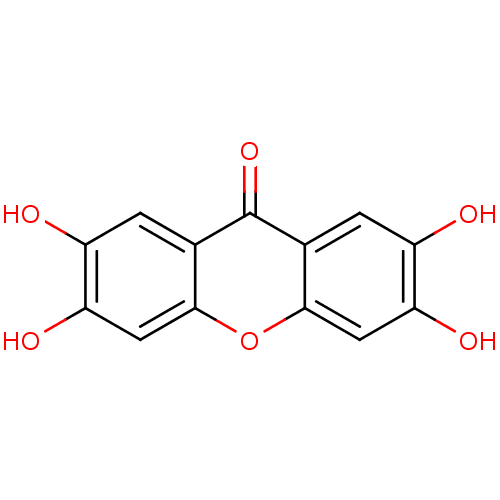
Affinity DataKi: 1.42E+4nMAssay Description:Inhibition of ACE by Lineweaver-Burk plotMore data for this Ligand-Target Pair
Affinity DataKi: 3.42E+4nMAssay Description:Inhibition of ACE by Lineweaver-Burk plotMore data for this Ligand-Target Pair
Affinity DataKi: 1.26E+5nMAssay Description:Inhibition of ACE by Lineweaver-Burk plotMore data for this Ligand-Target Pair
Affinity DataKi: 2.50E+5nMAssay Description:Inhibition of ACE by Lineweaver-Burk plotMore data for this Ligand-Target Pair
TargetXanthine dehydrogenase/oxidase(Homo sapiens (Human))
Kaohsiung Medical University
Curated by ChEMBL
Kaohsiung Medical University
Curated by ChEMBL
Affinity DataIC50: 1.90E+3nMAssay Description:Inhibition of xanthine oxidase activity assessed as uric acid formation pretreated for 15 mins before substrate addition measured after 5 minsMore data for this Ligand-Target Pair
TargetXanthine dehydrogenase/oxidase(Homo sapiens (Human))
Kaohsiung Medical University
Curated by ChEMBL
Kaohsiung Medical University
Curated by ChEMBL
Affinity DataIC50: 2.00E+3nMAssay Description:Inhibition of xanthine oxidase (unknown origin)More data for this Ligand-Target Pair
TargetXanthine dehydrogenase/oxidase(Homo sapiens (Human))
Kaohsiung Medical University
Curated by ChEMBL
Kaohsiung Medical University
Curated by ChEMBL
Affinity DataIC50: 2.00E+3nMAssay Description:Inhibition of xanthine oxidase (unknown origin)More data for this Ligand-Target Pair
Affinity DataIC50: 2.80E+3nMAssay Description:Inhibitory effect of compound on the release of Beta-glucuronidase in rat neutrophils stimulated with fMLP/CBMore data for this Ligand-Target Pair
Affinity DataIC50: 5.90E+3nMAssay Description:Inhibitory effect of compound on the release of Beta-glucuronidase in rat neutrophils stimulated with fMLP/CBMore data for this Ligand-Target Pair
Affinity DataIC50: 7.80E+3nMAssay Description:Inhibitory effect of compound on the release of Beta-glucuronidase in rat neutrophils stimulated with fMLP/CBMore data for this Ligand-Target Pair
Affinity DataIC50: 9.00E+3nMAssay Description:Inhibitory effect of compound on the release of lysozyme in rat neutrophils stimulated with fMLP/CBMore data for this Ligand-Target Pair
Affinity DataIC50: 9.70E+3nMAssay Description:Inhibitory effect of compound on the release of lysozyme in rat neutrophils stimulated with fMLP/CBMore data for this Ligand-Target Pair
Affinity DataIC50: 1.37E+4nMAssay Description:Inhibitory effect of compound on the release of Beta-glucuronidase in rat neutrophils stimulated with fMLP/CBMore data for this Ligand-Target Pair
Affinity DataIC50: 1.77E+4nMAssay Description:Inhibitory effect of compound on the release of lysozyme in rat neutrophils stimulated with fMLP/CBMore data for this Ligand-Target Pair
TargetXanthine dehydrogenase/oxidase(Homo sapiens (Human))
Kaohsiung Medical University
Curated by ChEMBL
Kaohsiung Medical University
Curated by ChEMBL
Affinity DataIC50: 2.13E+4nMAssay Description:Inhibition of xanthine oxidase (unknown origin)More data for this Ligand-Target Pair
Affinity DataIC50: 2.63E+4nMAssay Description:Inhibitory effect of compound on the release of lysozyme in rat neutrophils stimulated with fMLP/CBMore data for this Ligand-Target Pair
Affinity DataIC50: >3.00E+4nMAssay Description:Inhibitory effect of compound on the release of lysozyme in rat neutrophils stimulated with fMLP/CBMore data for this Ligand-Target Pair
Affinity DataIC50: >3.00E+4nMAssay Description:Inhibitory effect of compound on the release of lysozyme in rat neutrophils stimulated with fMLP/CBMore data for this Ligand-Target Pair
Affinity DataIC50: >3.00E+4nMAssay Description:Inhibitory effect of compound on the release of lysozyme in rat neutrophils stimulated with fMLP/CBMore data for this Ligand-Target Pair
Affinity DataIC50: >3.00E+4nMAssay Description:Inhibitory effect of compound on the release of Beta-glucuronidase in rat neutrophils stimulated with fMLP/CBMore data for this Ligand-Target Pair
Affinity DataIC50: >3.00E+4nMAssay Description:Inhibitory effect of compound on the release of Beta-glucuronidase in rat neutrophils stimulated with fMLP/CBMore data for this Ligand-Target Pair
Affinity DataIC50: >3.00E+4nMAssay Description:Inhibitory effect of compound on the release of lysozyme in rat neutrophils stimulated with fMLP/CBMore data for this Ligand-Target Pair
Affinity DataIC50: >3.00E+4nMAssay Description:Inhibitory effect of compound on the release of Beta-glucuronidase in rat neutrophils stimulated with fMLP/CBMore data for this Ligand-Target Pair
Affinity DataIC50: >3.00E+4nMAssay Description:Inhibitory effect of compound on the release of Beta-glucuronidase in rat neutrophils stimulated with fMLP/CBMore data for this Ligand-Target Pair
Affinity DataIC50: 3.54E+4nMAssay Description:Inhibition of ACEMore data for this Ligand-Target Pair
TargetXanthine dehydrogenase/oxidase(Homo sapiens (Human))
Kaohsiung Medical University
Curated by ChEMBL
Kaohsiung Medical University
Curated by ChEMBL
Affinity DataIC50: 3.78E+4nMAssay Description:Inhibition of xanthine oxidase activity assessed as uric acid formation pretreated for 15 mins before substrate addition measured after 5 minsMore data for this Ligand-Target Pair
TargetXanthine dehydrogenase/oxidase(Homo sapiens (Human))
Kaohsiung Medical University
Curated by ChEMBL
Kaohsiung Medical University
Curated by ChEMBL
Affinity DataIC50: 4.21E+4nMAssay Description:Inhibition of xanthine oxidase (unknown origin)More data for this Ligand-Target Pair
TargetXanthine dehydrogenase/oxidase(Homo sapiens (Human))
Kaohsiung Medical University
Curated by ChEMBL
Kaohsiung Medical University
Curated by ChEMBL
Affinity DataIC50: 4.21E+4nMAssay Description:Inhibition of xanthine oxidase (unknown origin)More data for this Ligand-Target Pair
TargetXanthine dehydrogenase/oxidase(Homo sapiens (Human))
Kaohsiung Medical University
Curated by ChEMBL
Kaohsiung Medical University
Curated by ChEMBL
Affinity DataIC50: 4.43E+4nMAssay Description:Inhibition of xanthine oxidase activity assessed as uric acid formation pretreated for 15 mins before substrate addition measured after 5 minsMore data for this Ligand-Target Pair
TargetXanthine dehydrogenase/oxidase(Homo sapiens (Human))
Kaohsiung Medical University
Curated by ChEMBL
Kaohsiung Medical University
Curated by ChEMBL
Affinity DataIC50: 4.73E+4nMAssay Description:Inhibition of xanthine oxidase activity assessed as uric acid formation pretreated for 15 mins before substrate addition measured after 5 minsMore data for this Ligand-Target Pair
TargetXanthine dehydrogenase/oxidase(Homo sapiens (Human))
Kaohsiung Medical University
Curated by ChEMBL
Kaohsiung Medical University
Curated by ChEMBL
Affinity DataIC50: 5.35E+4nMAssay Description:Inhibition of xanthine oxidase activity assessed as uric acid formation pretreated for 15 mins before substrate addition measured after 5 minsMore data for this Ligand-Target Pair
TargetXanthine dehydrogenase/oxidase(Homo sapiens (Human))
Kaohsiung Medical University
Curated by ChEMBL
Kaohsiung Medical University
Curated by ChEMBL
Affinity DataIC50: 5.65E+4nMAssay Description:Inhibition of xanthine oxidase activity assessed as uric acid formation pretreated for 15 mins before substrate addition measured after 5 minsMore data for this Ligand-Target Pair
TargetXanthine dehydrogenase/oxidase(Homo sapiens (Human))
Kaohsiung Medical University
Curated by ChEMBL
Kaohsiung Medical University
Curated by ChEMBL
Affinity DataIC50: 6.30E+4nMAssay Description:Inhibition of xanthine oxidase activity assessed as uric acid formation pretreated for 15 mins before substrate addition measured after 5 minsMore data for this Ligand-Target Pair
TargetXanthine dehydrogenase/oxidase(Homo sapiens (Human))
Kaohsiung Medical University
Curated by ChEMBL
Kaohsiung Medical University
Curated by ChEMBL
Affinity DataIC50: 6.55E+4nMAssay Description:Inhibition of xanthine oxidase activity assessed as uric acid formation pretreated for 15 mins before substrate addition measured after 5 minsMore data for this Ligand-Target Pair
TargetXanthine dehydrogenase/oxidase(Homo sapiens (Human))
Kaohsiung Medical University
Curated by ChEMBL
Kaohsiung Medical University
Curated by ChEMBL
Affinity DataIC50: 6.88E+4nMAssay Description:Inhibition of xanthine oxidase activity assessed as uric acid formation pretreated for 15 mins before substrate addition measured after 5 minsMore data for this Ligand-Target Pair
Affinity DataIC50: 6.92E+4nMAssay Description:Inhibition of ACEMore data for this Ligand-Target Pair
TargetXanthine dehydrogenase/oxidase(Homo sapiens (Human))
Kaohsiung Medical University
Curated by ChEMBL
Kaohsiung Medical University
Curated by ChEMBL
Affinity DataIC50: 7.29E+4nMAssay Description:Inhibition of xanthine oxidase activity assessed as uric acid formation pretreated for 15 mins before substrate addition measured after 5 minsMore data for this Ligand-Target Pair
TargetXanthine dehydrogenase/oxidase(Homo sapiens (Human))
Kaohsiung Medical University
Curated by ChEMBL
Kaohsiung Medical University
Curated by ChEMBL
Affinity DataIC50: 8.23E+4nMAssay Description:Inhibition of xanthine oxidase activity assessed as uric acid formation pretreated for 15 mins before substrate addition measured after 5 minsMore data for this Ligand-Target Pair
TargetXanthine dehydrogenase/oxidase(Homo sapiens (Human))
Kaohsiung Medical University
Curated by ChEMBL
Kaohsiung Medical University
Curated by ChEMBL
Affinity DataIC50: 9.19E+4nMAssay Description:Inhibition of xanthine oxidase activity assessed as uric acid formation pretreated for 15 mins before substrate addition measured after 5 minsMore data for this Ligand-Target Pair
TargetXanthine dehydrogenase/oxidase(Homo sapiens (Human))
Kaohsiung Medical University
Curated by ChEMBL
Kaohsiung Medical University
Curated by ChEMBL
Affinity DataIC50: 1.09E+5nMAssay Description:Inhibition of xanthine oxidase activity assessed as uric acid formation pretreated for 15 mins before substrate addition measured after 5 minsMore data for this Ligand-Target Pair
TargetXanthine dehydrogenase/oxidase(Homo sapiens (Human))
Kaohsiung Medical University
Curated by ChEMBL
Kaohsiung Medical University
Curated by ChEMBL
Affinity DataIC50: 1.31E+5nMAssay Description:Inhibition of xanthine oxidase (unknown origin)More data for this Ligand-Target Pair
Affinity DataIC50: 2.39E+5nMAssay Description:Inhibition of ACEMore data for this Ligand-Target Pair
Affinity DataIC50: 5.31E+5nMAssay Description:Inhibition of ACEMore data for this Ligand-Target Pair
Affinity DataIC50: 7.69E+5nMAssay Description:Inhibition of ACEMore data for this Ligand-Target Pair