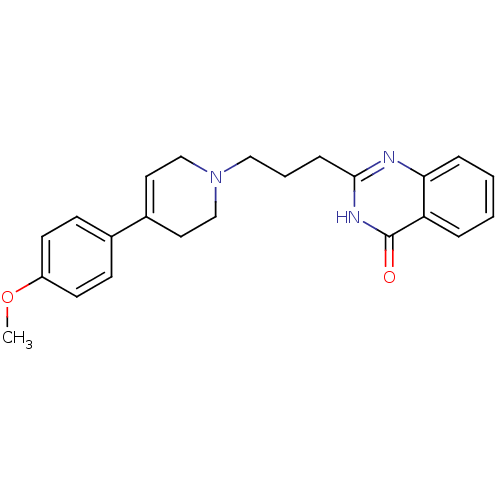
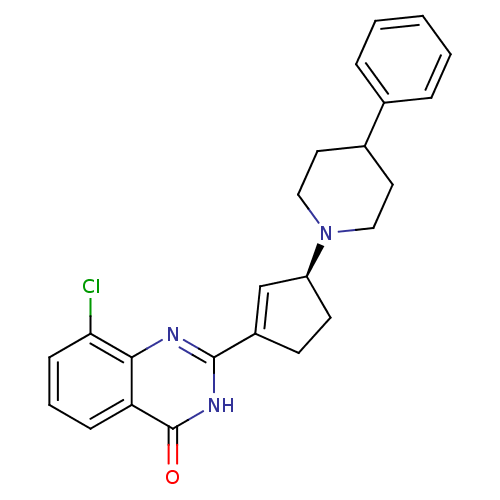
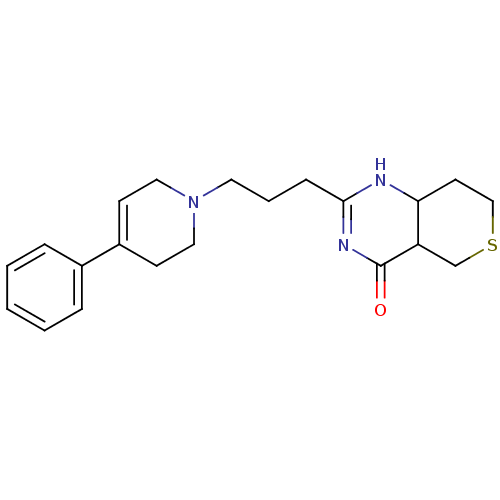
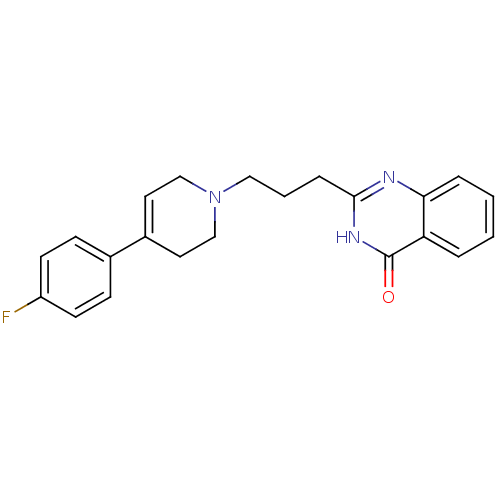
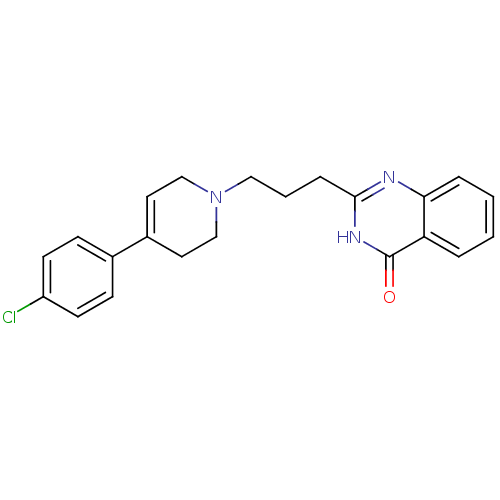
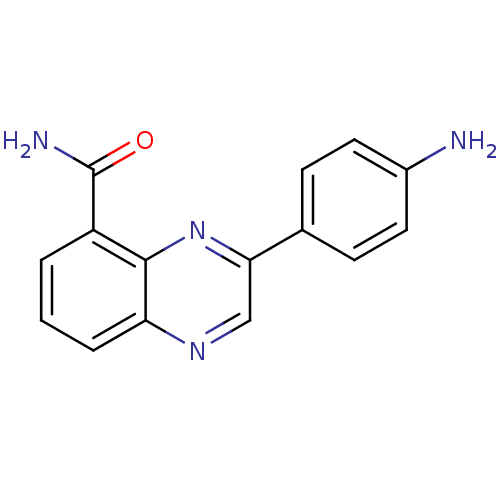
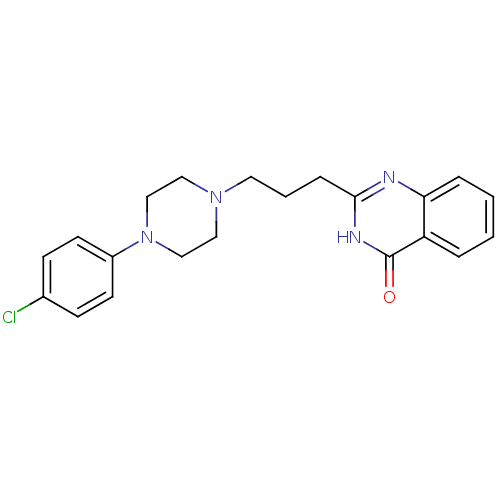
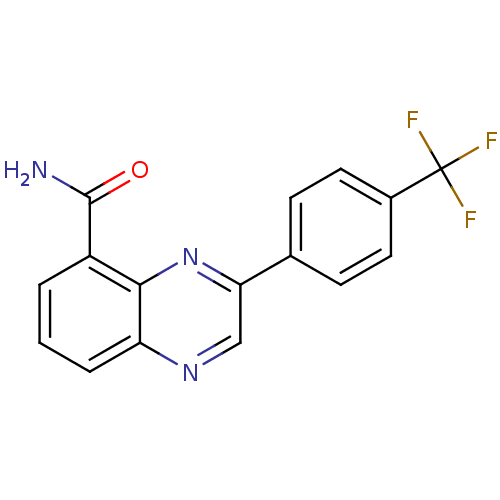
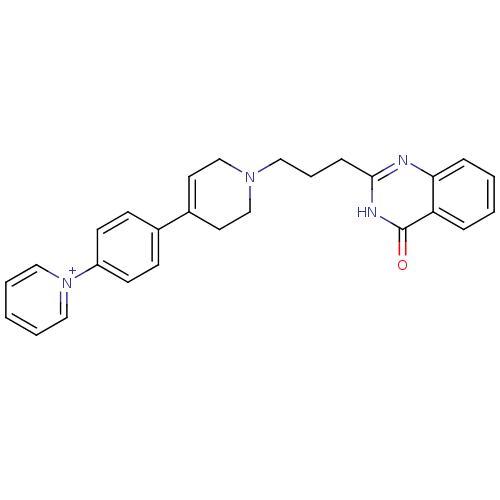
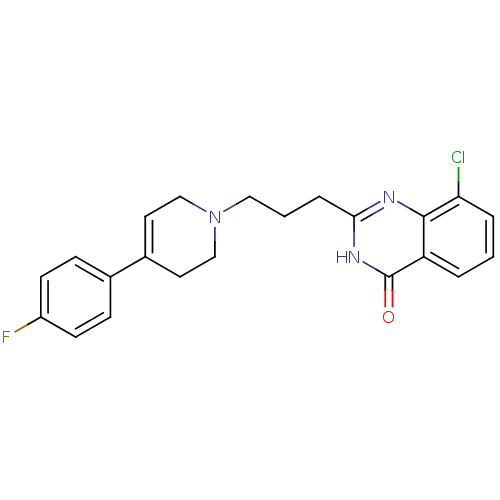
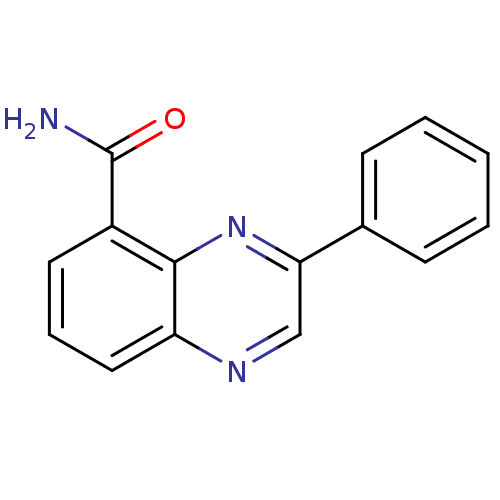
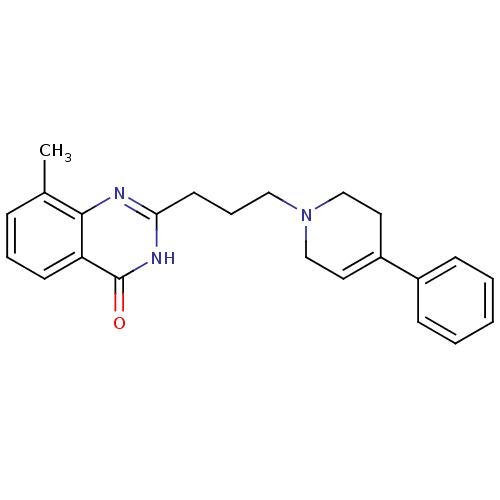
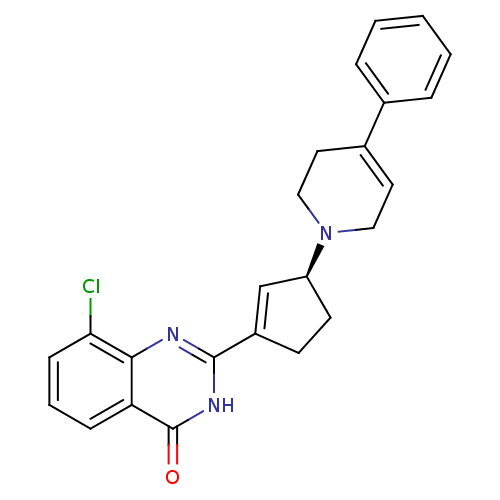
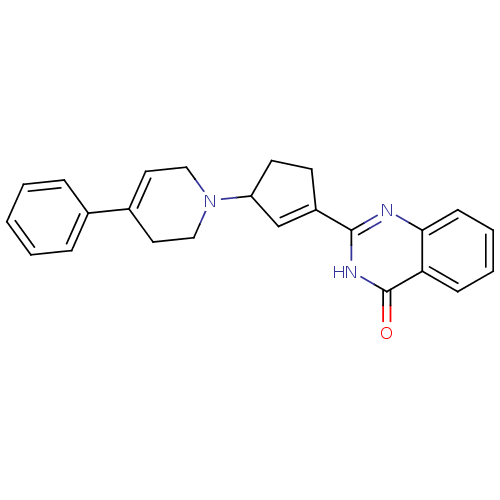
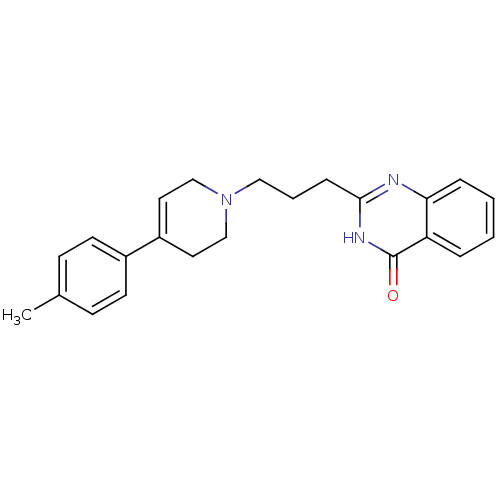
TargetNicotinamide N-methyltransferase(Mus musculus)
Shionogi Pharmaceutical Research Center
Curated by ChEMBL
Shionogi Pharmaceutical Research Center
Curated by ChEMBL
Affinity DataIC50: 0.150nMAssay Description:Inhibition of mouse recombinant NNMT using nicotinamide and SAM as substrate assessed as reduction in 1-methyl-nicotinamide formation incubated for 2...More data for this Ligand-Target Pair
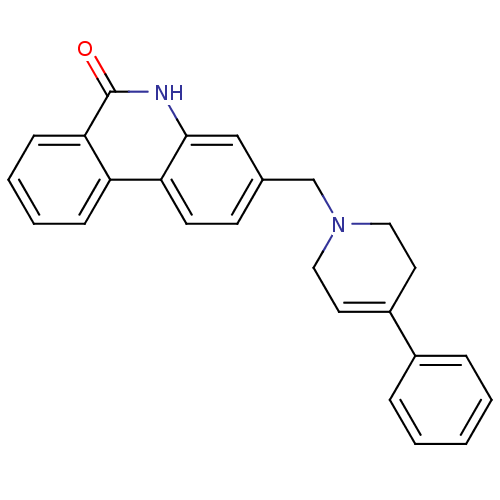
TargetNicotinamide N-methyltransferase(Homo sapiens (Human))
Shionogi Pharmaceutical Research Center
Curated by ChEMBL
Shionogi Pharmaceutical Research Center
Curated by ChEMBL
Affinity DataIC50: 0.150nMAssay Description:Inhibition of human recombinant NNMT using nicotinamide and SAM as substrate assessed as reduction in 1-methyl-nicotinamide formation incubated for 2...More data for this Ligand-Target Pair
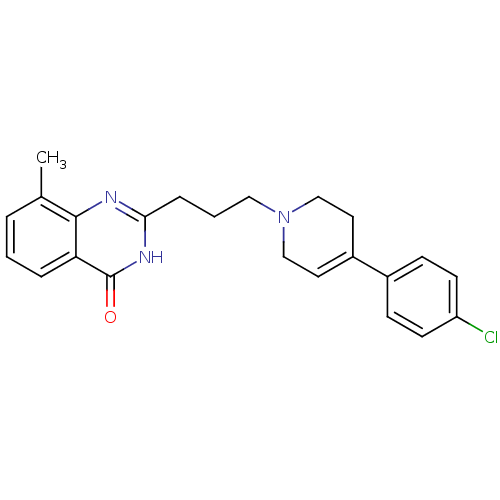
TargetNicotinamide N-methyltransferase(Homo sapiens (Human))
Shionogi Pharmaceutical Research Center
Curated by ChEMBL
Shionogi Pharmaceutical Research Center
Curated by ChEMBL
Affinity DataIC50: 0.400nMAssay Description:Inhibition of human recombinant NNMT using nicotinamide and SAM as substrate assessed as reduction in 1-methyl-nicotinamide formation incubated for 2...More data for this Ligand-Target Pair
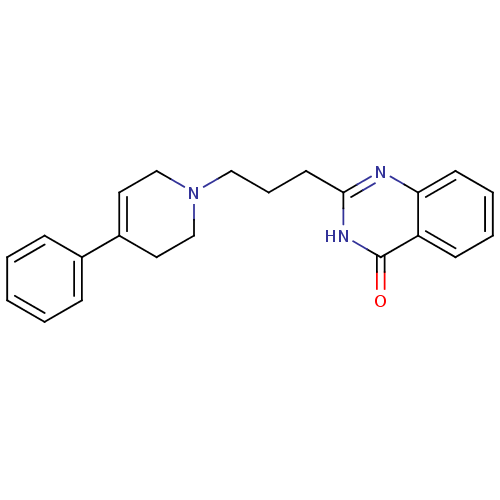
TargetNicotinamide N-methyltransferase(Homo sapiens (Human))
Shionogi Pharmaceutical Research Center
Curated by ChEMBL
Shionogi Pharmaceutical Research Center
Curated by ChEMBL
Affinity DataIC50: 0.470nMAssay Description:Inhibition of human recombinant NNMT using nicotinamide and SAM as substrate assessed as reduction in 1-methyl-nicotinamide formation incubated for 2...More data for this Ligand-Target Pair
TargetNicotinamide N-methyltransferase(Mus musculus)
Shionogi Pharmaceutical Research Center
Curated by ChEMBL
Shionogi Pharmaceutical Research Center
Curated by ChEMBL
Affinity DataIC50: 0.620nMAssay Description:Inhibition of mouse recombinant NNMT using nicotinamide and SAM as substrate assessed as reduction in 1-methyl-nicotinamide formation incubated for 2...More data for this Ligand-Target Pair
TargetNicotinamide N-methyltransferase(Homo sapiens (Human))
Shionogi Pharmaceutical Research Center
Curated by ChEMBL
Shionogi Pharmaceutical Research Center
Curated by ChEMBL
Affinity DataIC50: 0.930nMAssay Description:Inhibition of human recombinant NNMT using nicotinamide and SAM as substrate assessed as reduction in 1-methyl-nicotinamide formation incubated for 2...More data for this Ligand-Target Pair
TargetNicotinamide N-methyltransferase(Mus musculus)
Shionogi Pharmaceutical Research Center
Curated by ChEMBL
Shionogi Pharmaceutical Research Center
Curated by ChEMBL
Affinity DataIC50: 0.950nMAssay Description:Inhibition of mouse recombinant NNMT using nicotinamide and SAM as substrate assessed as reduction in 1-methyl-nicotinamide formation incubated for 2...More data for this Ligand-Target Pair
TargetNicotinamide N-methyltransferase(Homo sapiens (Human))
Shionogi Pharmaceutical Research Center
Curated by ChEMBL
Shionogi Pharmaceutical Research Center
Curated by ChEMBL
Affinity DataIC50: 1.10nMAssay Description:Inhibition of human recombinant NNMT using nicotinamide and SAM as substrate assessed as reduction in 1-methyl-nicotinamide formation incubated for 2...More data for this Ligand-Target Pair
TargetNicotinamide N-methyltransferase(Mus musculus)
Shionogi Pharmaceutical Research Center
Curated by ChEMBL
Shionogi Pharmaceutical Research Center
Curated by ChEMBL
Affinity DataIC50: 1.20nMAssay Description:Inhibition of mouse recombinant NNMT using nicotinamide and SAM as substrate assessed as reduction in 1-methyl-nicotinamide formation incubated for 2...More data for this Ligand-Target Pair
TargetNicotinamide N-methyltransferase(Mus musculus)
Shionogi Pharmaceutical Research Center
Curated by ChEMBL
Shionogi Pharmaceutical Research Center
Curated by ChEMBL
Affinity DataIC50: 1.30nMAssay Description:Inhibition of mouse recombinant NNMT using nicotinamide and SAM as substrate assessed as reduction in 1-methyl-nicotinamide formation incubated for 2...More data for this Ligand-Target Pair
TargetNicotinamide N-methyltransferase(Homo sapiens (Human))
Shionogi Pharmaceutical Research Center
Curated by ChEMBL
Shionogi Pharmaceutical Research Center
Curated by ChEMBL
Affinity DataIC50: 1.90nMAssay Description:Inhibition of human recombinant NNMT using nicotinamide and SAM as substrate assessed as reduction in 1-methyl-nicotinamide formation incubated for 2...More data for this Ligand-Target Pair
TargetNicotinamide N-methyltransferase(Mus musculus)
Shionogi Pharmaceutical Research Center
Curated by ChEMBL
Shionogi Pharmaceutical Research Center
Curated by ChEMBL
Affinity DataIC50: 2nMAssay Description:Inhibition of mouse recombinant NNMT using nicotinamide and SAM as substrate assessed as reduction in 1-methyl-nicotinamide formation incubated for 2...More data for this Ligand-Target Pair
Affinity DataIC50: 3nMpH: 8.0 T: 2°CAssay Description:To assess the inhibitory activity of novel inhibitors, the PARP enzyme assay was carried out in reaction mixture consisting of activated salmon teste...More data for this Ligand-Target Pair
TargetNicotinamide N-methyltransferase(Homo sapiens (Human))
Shionogi Pharmaceutical Research Center
Curated by ChEMBL
Shionogi Pharmaceutical Research Center
Curated by ChEMBL
Affinity DataIC50: 5.80nMAssay Description:Inhibition of human recombinant NNMT using nicotinamide and SAM as substrate assessed as reduction in 1-methyl-nicotinamide formation incubated for 2...More data for this Ligand-Target Pair
Affinity DataIC50: 6nMAssay Description:In vitro inhibitory concentration against human recombinant Poly (ADP-ribose) polymerase 1More data for this Ligand-Target Pair
Affinity DataIC50: 7nMT: 2°CAssay Description:To assess the inhibitory activity of novel inhibitors, the PARP enzyme assay was carried out in reaction mixture consisting of activated salmon teste...More data for this Ligand-Target Pair
Affinity DataIC50: 8nMT: 2°CAssay Description:To assess the inhibitory activity of novel inhibitors, the PARP enzyme assay was carried out in reaction mixture consisting of activated salmon teste...More data for this Ligand-Target Pair
Affinity DataIC50: 8nMT: 2°CAssay Description:To assess the inhibitory activity of novel inhibitors, the PARP enzyme assay was carried out in reaction mixture consisting of activated salmon teste...More data for this Ligand-Target Pair
Affinity DataIC50: 8nMAssay Description:Inhibition of human PARP1More data for this Ligand-Target Pair
Affinity DataIC50: 8.30nMAssay Description:In vitro inhibitory concentration against human recombinant Poly (ADP-ribose) polymerase 1More data for this Ligand-Target Pair
Affinity DataIC50: 8.70nMAssay Description:Inhibition of human PARP1More data for this Ligand-Target Pair
Affinity DataIC50: 8.90nMAssay Description:In vitro inhibitory concentration against Poly(ADP-ribose) polymerase 1More data for this Ligand-Target Pair
Affinity DataIC50: 8.90nMAssay Description:In vitro inhibitory concentration against human recombinant Poly (ADP-ribose) polymerase 1More data for this Ligand-Target Pair
Affinity DataIC50: 8.90nMAssay Description:Inhibition of human PARP1More data for this Ligand-Target Pair
Affinity DataIC50: 9nMT: 2°CAssay Description:To assess the inhibitory activity of novel inhibitors, the PARP enzyme assay was carried out in reaction mixture consisting of activated salmon teste...More data for this Ligand-Target Pair
Affinity DataIC50: 11nMpH: 8.0 T: 2°CAssay Description:To assess the inhibitory activity of novel inhibitors, the PARP enzyme assay was carried out in reaction mixture consisting of activated salmon teste...More data for this Ligand-Target Pair
Affinity DataIC50: 11nMT: 2°CAssay Description:To assess the inhibitory activity of novel inhibitors, the PARP enzyme assay was carried out in reaction mixture consisting of activated salmon teste...More data for this Ligand-Target Pair
Affinity DataIC50: 12nMAssay Description:Inhibition of human PARP1More data for this Ligand-Target Pair
Affinity DataIC50: 12nMAssay Description:In vitro inhibitory concentration against human recombinant Poly (ADP-ribose) polymerase 1More data for this Ligand-Target Pair
TargetNicotinamide N-methyltransferase(Homo sapiens (Human))
Shionogi Pharmaceutical Research Center
Curated by ChEMBL
Shionogi Pharmaceutical Research Center
Curated by ChEMBL
Affinity DataIC50: 12nMAssay Description:Inhibition of human recombinant NNMT using nicotinamide and SAM as substrate assessed as reduction in 1-methyl-nicotinamide formation incubated for 2...More data for this Ligand-Target Pair
Affinity DataIC50: 12nMAssay Description:In vitro inhibitory concentration against human recombinant Poly (ADP-ribose) polymerase 1More data for this Ligand-Target Pair
Affinity DataIC50: 12nMAssay Description:In vitro inhibitory concentration against human recombinant Poly (ADP-ribose) polymerase 1More data for this Ligand-Target Pair
Affinity DataIC50: 13nMpH: 8.0 T: 2°CAssay Description:To assess the inhibitory activity of novel inhibitors, the PARP enzyme assay was carried out in reaction mixture consisting of activated salmon teste...More data for this Ligand-Target Pair
TargetNicotinamide N-methyltransferase(Mus musculus)
Shionogi Pharmaceutical Research Center
Curated by ChEMBL
Shionogi Pharmaceutical Research Center
Curated by ChEMBL
Affinity DataIC50: 13nMAssay Description:Inhibition of mouse recombinant NNMT using nicotinamide and SAM as substrate assessed as reduction in 1-methyl-nicotinamide formation incubated for 2...More data for this Ligand-Target Pair
Affinity DataIC50: 13nMAssay Description:In vitro inhibitory concentration against human recombinant Poly (ADP-ribose) polymerase 1More data for this Ligand-Target Pair
Affinity DataIC50: 13nMAssay Description:Inhibition of human PARP1More data for this Ligand-Target Pair
Affinity DataIC50: 14nMT: 2°CAssay Description:To assess the inhibitory activity of novel inhibitors, the PARP enzyme assay was carried out in reaction mixture consisting of activated salmon teste...More data for this Ligand-Target Pair
TargetNicotinamide N-methyltransferase(Homo sapiens (Human))
Shionogi Pharmaceutical Research Center
Curated by ChEMBL
Shionogi Pharmaceutical Research Center
Curated by ChEMBL
Affinity DataIC50: 14nMAssay Description:Inhibition of human recombinant NNMT using nicotinamide and SAM as substrate assessed as reduction in 1-methyl-nicotinamide formation incubated for 2...More data for this Ligand-Target Pair
Affinity DataIC50: 14nMAssay Description:In vitro inhibitory concentration against human recombinant Poly (ADP-ribose) polymerase 1More data for this Ligand-Target Pair
TargetNicotinamide N-methyltransferase(Homo sapiens (Human))
Shionogi Pharmaceutical Research Center
Curated by ChEMBL
Shionogi Pharmaceutical Research Center
Curated by ChEMBL
Affinity DataIC50: 14nMAssay Description:Inhibition of human recombinant NNMT using nicotinamide and SAM as substrate assessed as reduction in 1-methyl-nicotinamide formation incubated for 2...More data for this Ligand-Target Pair
Affinity DataIC50: 15nMAssay Description:Inhibition of human PARP1More data for this Ligand-Target Pair
Affinity DataIC50: 16nMAssay Description:In vitro inhibitory concentration against Poly(ADP-ribose) polymerase 1More data for this Ligand-Target Pair
Affinity DataIC50: 16nMAssay Description:Inhibition of human PARP1More data for this Ligand-Target Pair
Affinity DataIC50: 16nMAssay Description:Inhibition of human PARP1More data for this Ligand-Target Pair
Affinity DataIC50: 16nMAssay Description:In vitro inhibitory concentration against human recombinant Poly (ADP-ribose) polymerase 1More data for this Ligand-Target Pair
Affinity DataIC50: 16nMAssay Description:Inhibition of human PARP1More data for this Ligand-Target Pair
Affinity DataIC50: 16nMAssay Description:In vitro inhibitory concentration against human recombinant Poly (ADP-ribose) polymerase 1More data for this Ligand-Target Pair
Affinity DataIC50: 16nMAssay Description:Inhibition of human PARP1More data for this Ligand-Target Pair
Affinity DataIC50: 16nMpH: 8.0 T: 2°CAssay Description:To assess the inhibitory activity of novel inhibitors, the PARP enzyme assay was carried out in reaction mixture consisting of activated salmon teste...More data for this Ligand-Target Pair
Affinity DataIC50: 17nMAssay Description:In vitro inhibitory concentration against human recombinant Poly (ADP-ribose) polymerase 1More data for this Ligand-Target Pair

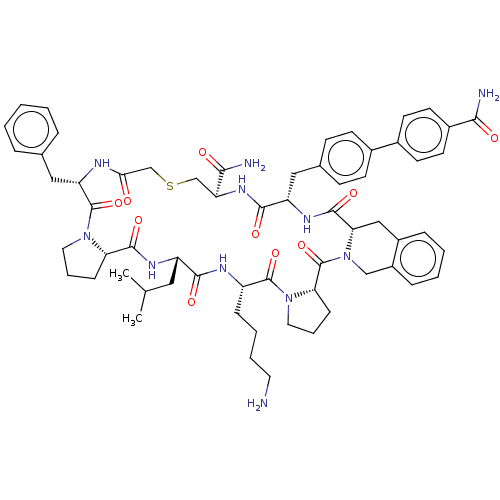





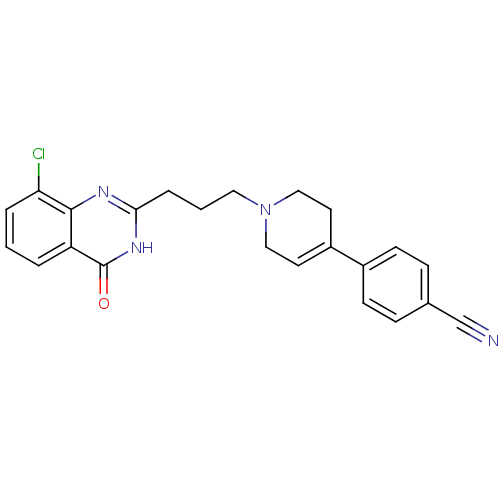

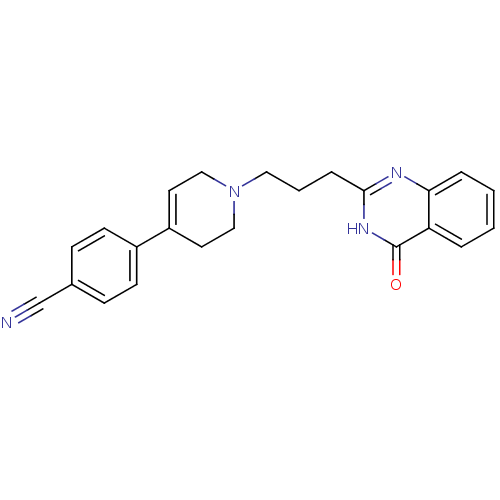
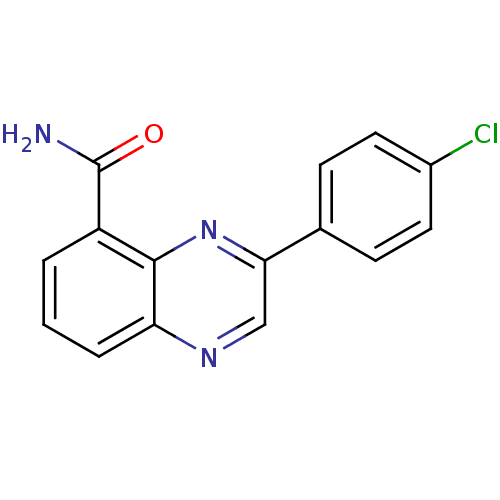
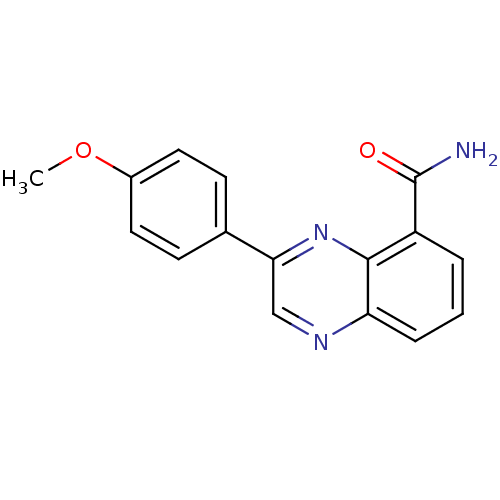
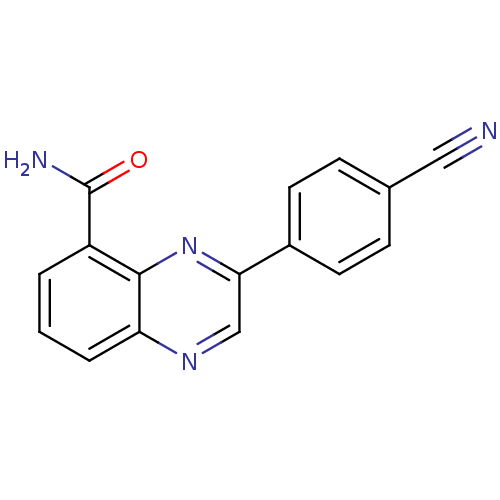
 3D Structure (crystal)
3D Structure (crystal)