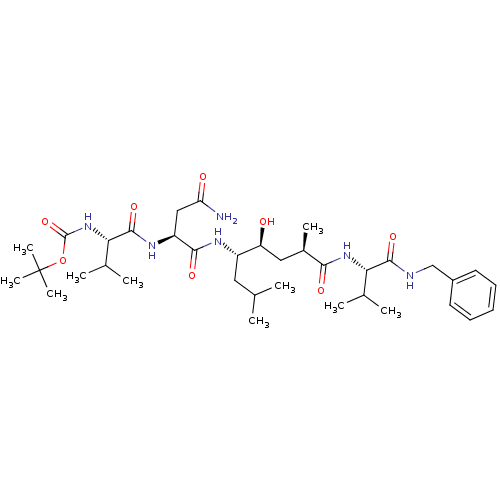
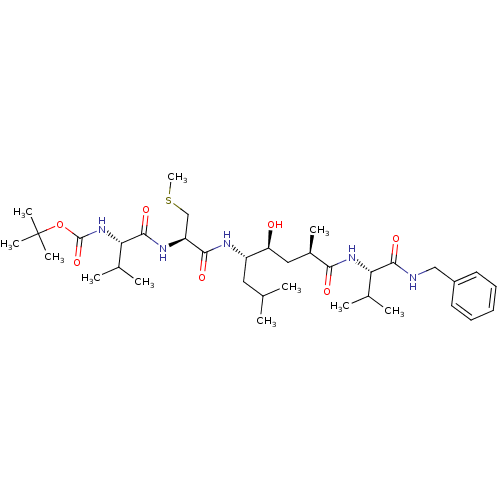
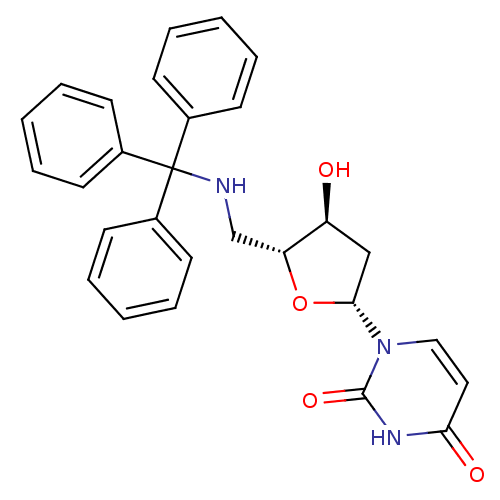
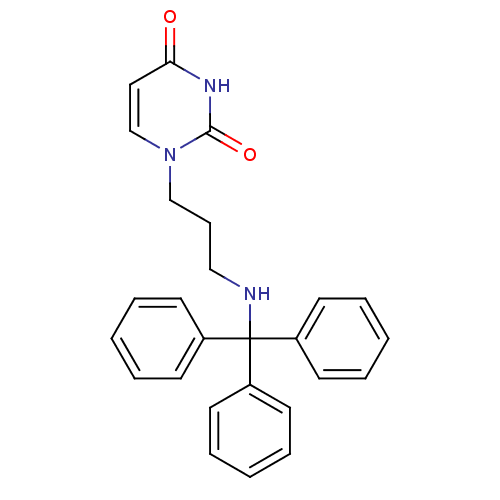
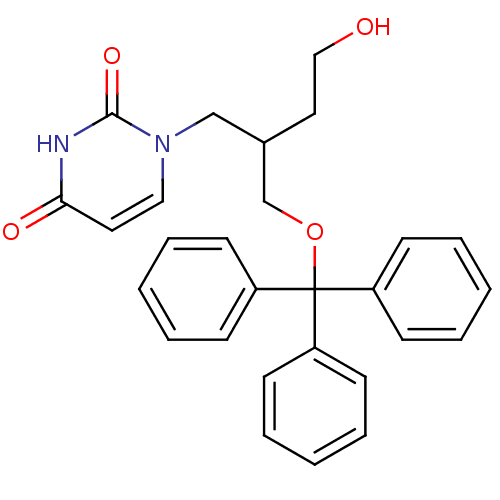
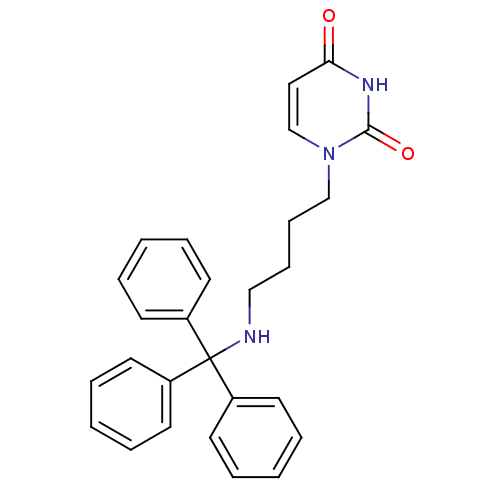
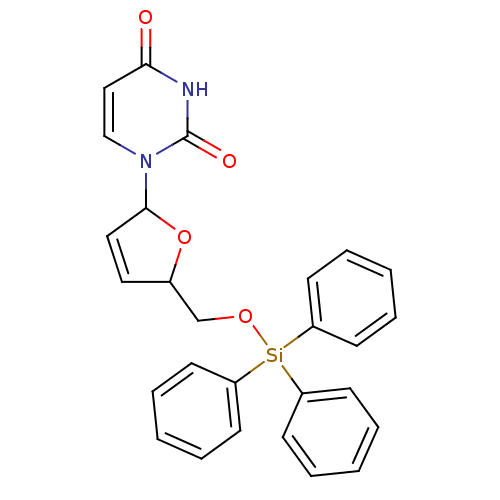
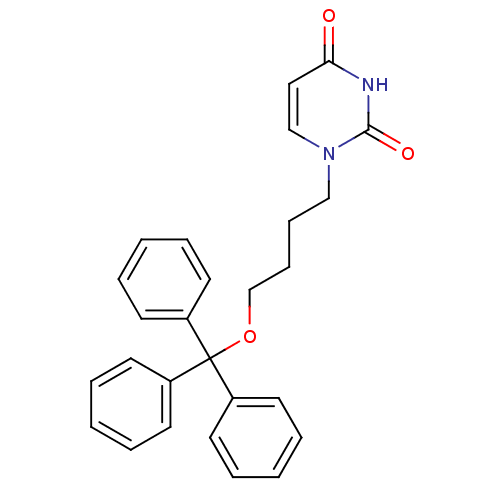
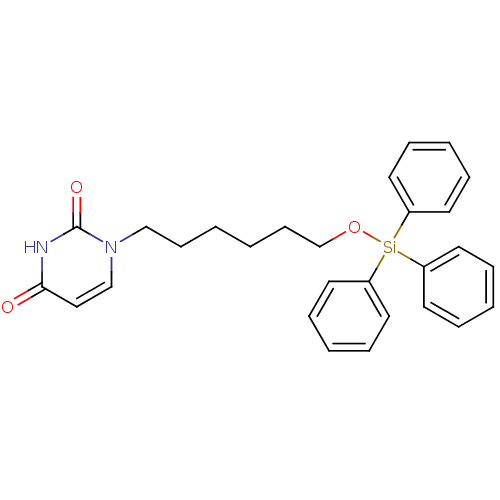
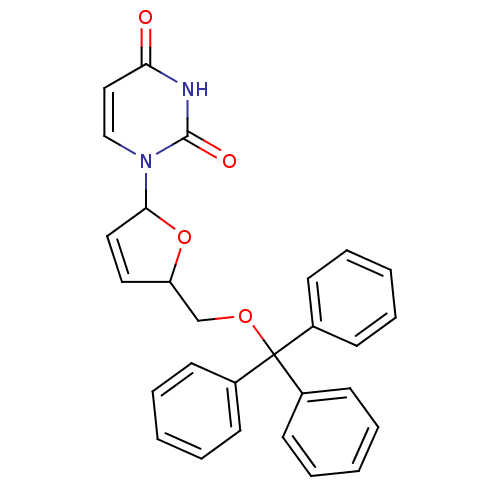
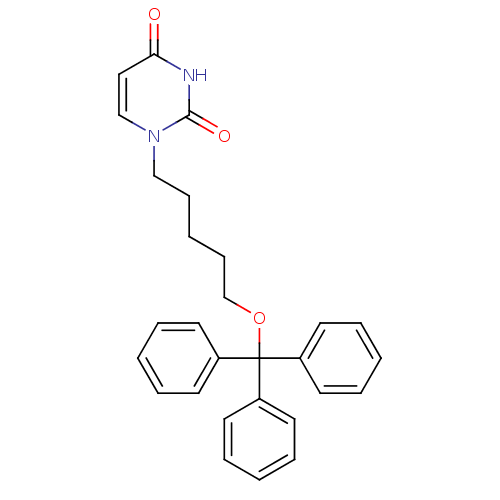
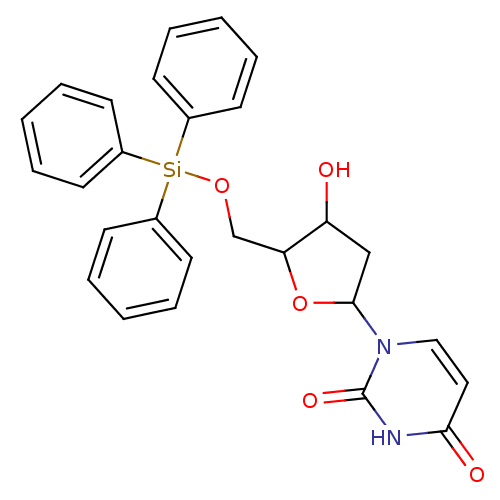
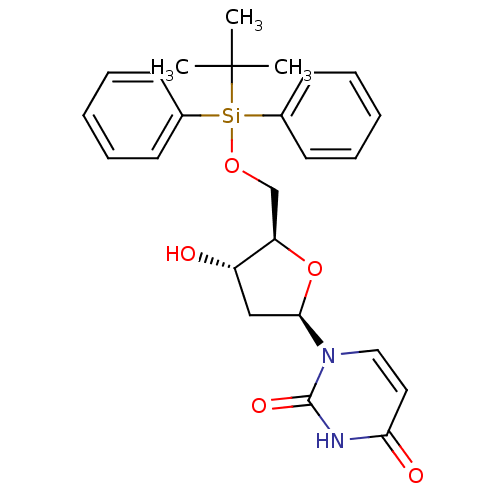
Target5-hydroxytryptamine receptor 4(Homo sapiens (Human))
Drug Discovery Laboratory
Curated by ChEMBL
Drug Discovery Laboratory
Curated by ChEMBL
Affinity DataKi: 0.0398nMAssay Description:Displacement of [3H]GR113808 from human 5-HT4B receptor expressed in HEK293 cells after 1 hr by liquid scintillation counting analysisMore data for this Ligand-Target Pair
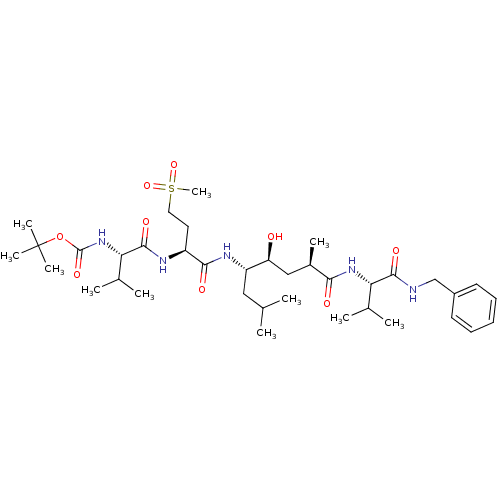
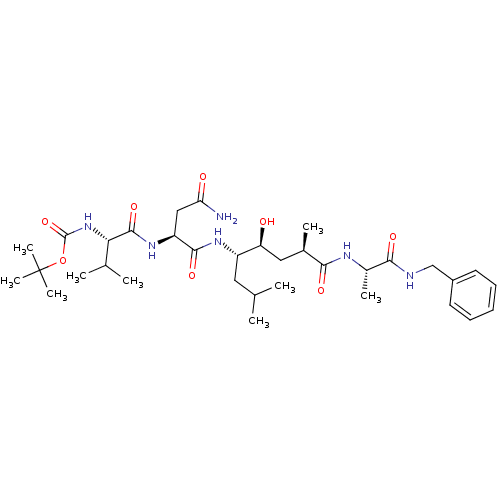
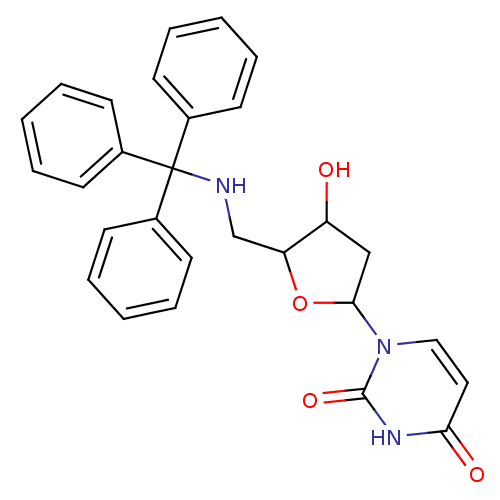
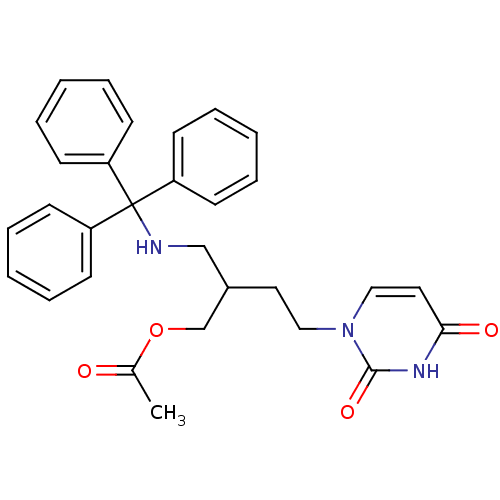
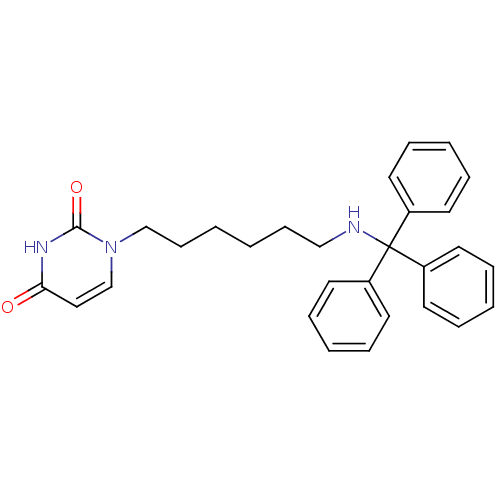
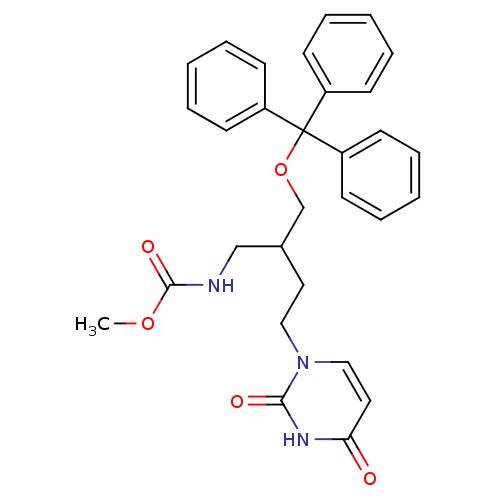
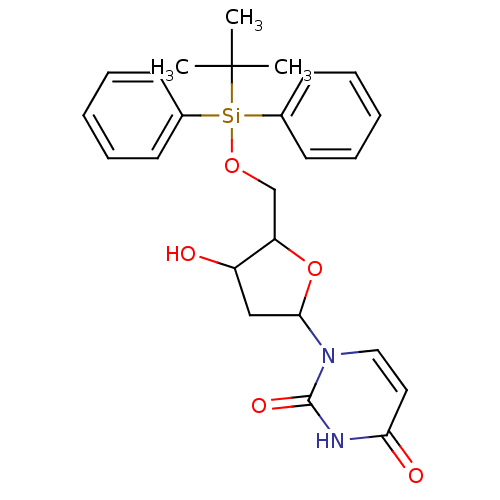
Target5-hydroxytryptamine receptor 4(Homo sapiens (Human))
Drug Discovery Laboratory
Curated by ChEMBL
Drug Discovery Laboratory
Curated by ChEMBL
Affinity DataKi: 0.100nMAssay Description:Displacement of [3H]GR113808 from human 5-HT4B receptor expressed in HEK293 cells after 1 hr by liquid scintillation counting analysisMore data for this Ligand-Target Pair
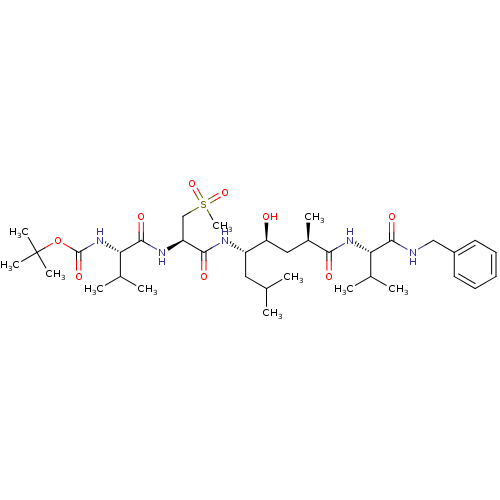
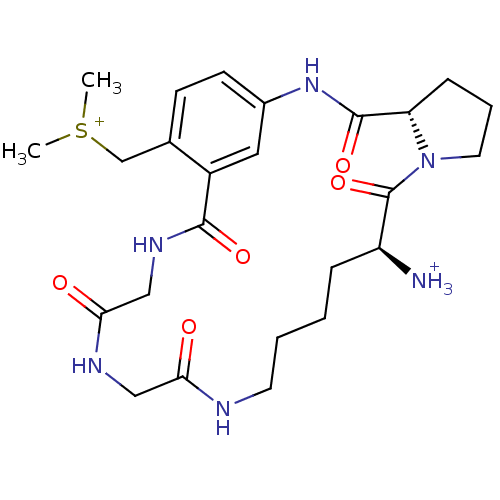
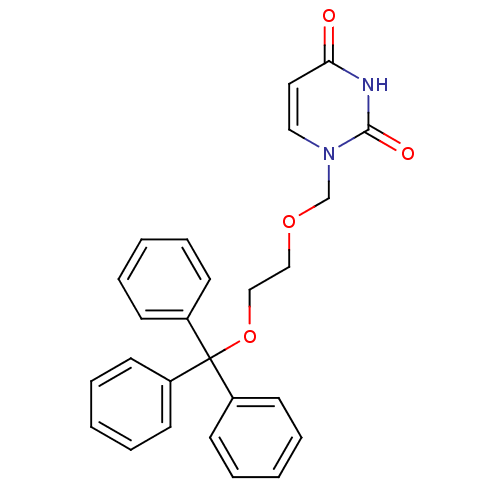
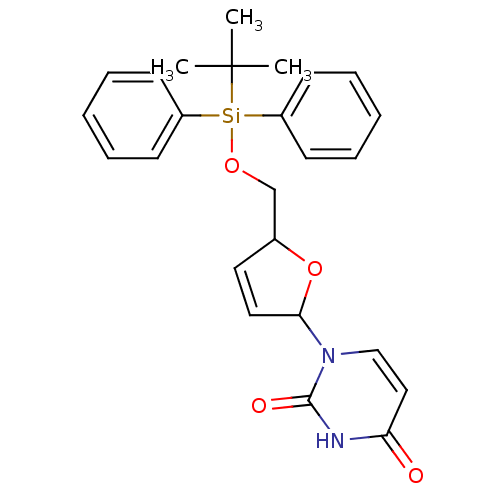
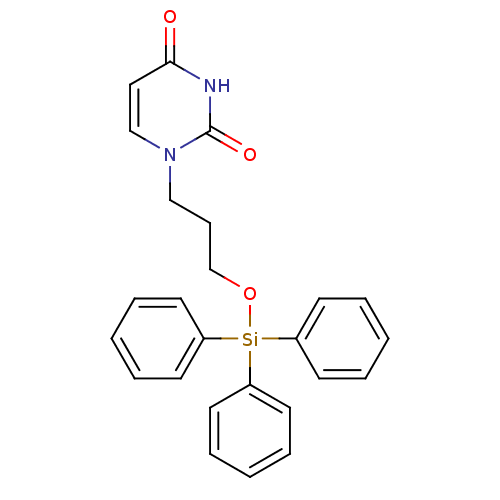
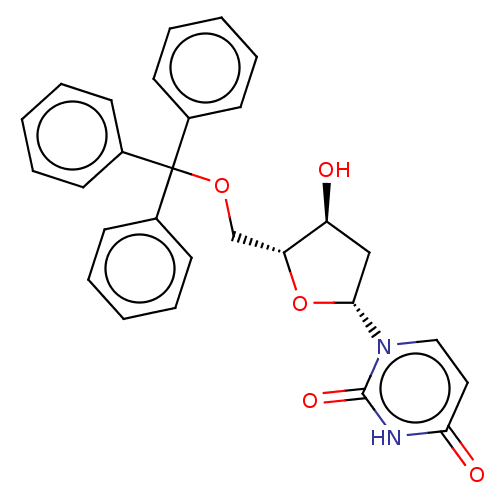
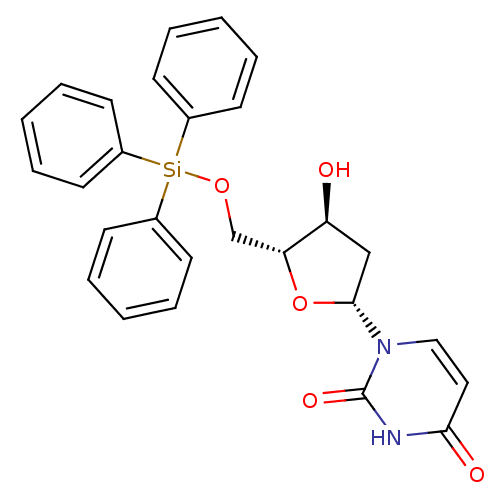
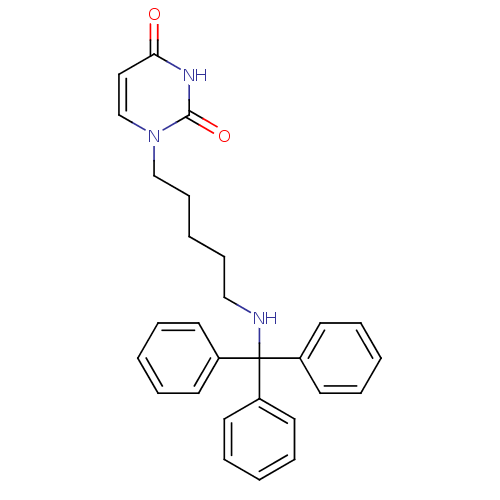
Target5-hydroxytryptamine receptor 4(Homo sapiens (Human))
Drug Discovery Laboratory
Curated by ChEMBL
Drug Discovery Laboratory
Curated by ChEMBL
Affinity DataKi: 0.126nMAssay Description:Displacement of [3H]GR113808 from human 5-HT4B receptor expressed in HEK293 cells after 1 hr by liquid scintillation counting analysisMore data for this Ligand-Target Pair
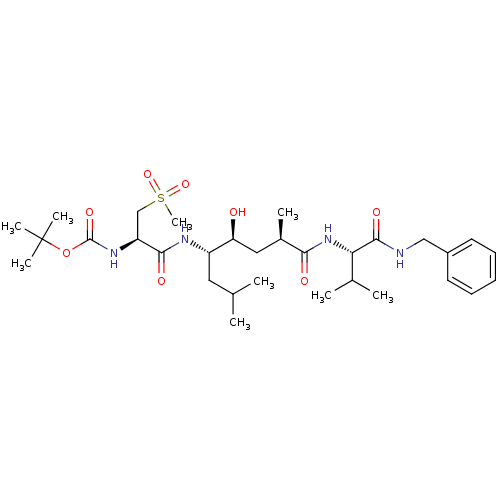
Affinity DataKi: 1.60nM ΔG°: -52.2kJ/molepH: 4.5 T: 2°CAssay Description:Enzyme activities were assayed by monitoring the hydrolysis of substrate in the presence or absence of inhibitor compounds. The hydrolysis was record...More data for this Ligand-Target Pair
Affinity DataKi: 2.5nM ΔG°: -51.1kJ/molepH: 4.5 T: 2°CAssay Description:Enzyme activities were assayed by monitoring the hydrolysis of substrate in the presence or absence of inhibitor compounds. The hydrolysis was record...More data for this Ligand-Target Pair
Affinity DataKi: 5.90nM ΔG°: -48.9kJ/molepH: 4.5 T: 2°CAssay Description:Enzyme activities were assayed by monitoring the hydrolysis of substrate in the presence or absence of inhibitor compounds. The hydrolysis was record...More data for this Ligand-Target Pair
Affinity DataKi: 8nM ΔG°: -48.1kJ/molepH: 4.5 T: 2°CAssay Description:Enzyme activities were assayed by monitoring the hydrolysis of substrate in the presence or absence of inhibitor compounds. The hydrolysis was record...More data for this Ligand-Target Pair
Affinity DataKi: 9.40nM ΔG°: -47.7kJ/molepH: 4.5 T: 2°CAssay Description:Enzyme activities were assayed by monitoring the hydrolysis of substrate in the presence or absence of inhibitor compounds. The hydrolysis was record...More data for this Ligand-Target Pair
Affinity DataKi: 36nM ΔG°: -44.2kJ/molepH: 4.5 T: 2°CAssay Description:Enzyme activities were assayed by monitoring the hydrolysis of substrate in the presence or absence of inhibitor compounds. The hydrolysis was record...More data for this Ligand-Target Pair
Affinity DataKi: 50.1nM ΔG°: -43.3kJ/molepH: 4.5 T: 2°CAssay Description:Enzyme activities were assayed by monitoring the hydrolysis of substrate in the presence or absence of inhibitor compounds. The hydrolysis was record...More data for this Ligand-Target Pair
Affinity DataKi: 61.4nM ΔG°: -42.8kJ/molepH: 4.5 T: 2°CAssay Description:Enzyme activities were assayed by monitoring the hydrolysis of substrate in the presence or absence of inhibitor compounds. The hydrolysis was record...More data for this Ligand-Target Pair
Affinity DataKi: 85nMAssay Description:Inhibitory activity against hydrolysis of Gly-Pro-pNa by CD26 (dipeptidylpeptidase 4) purified from CEM H01 cellsMore data for this Ligand-Target Pair
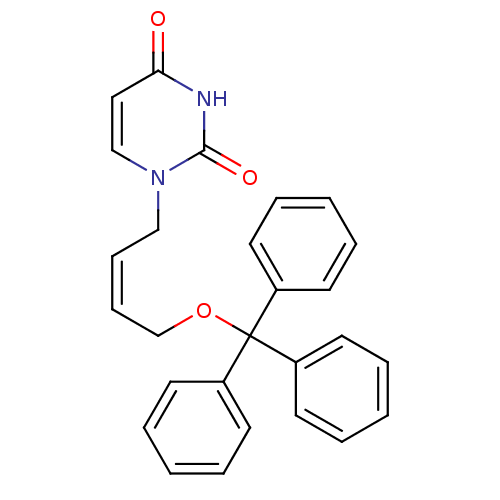
TargetDeoxyuridine 5'-triphosphate nucleotidohydrolase(Plasmodium falciparum)
Cardiff University
Curated by ChEMBL
Cardiff University
Curated by ChEMBL
Affinity DataKi: 200nMAssay Description:Inhibition of Plasmodium falciparum dUTPaseMore data for this Ligand-Target Pair
TargetDeoxyuridine 5'-triphosphate nucleotidohydrolase(Plasmodium falciparum)
Cardiff University
Curated by ChEMBL
Cardiff University
Curated by ChEMBL
Affinity DataKi: 200nMAssay Description:Inhibition of Plasmodium falciparum dUTPaseMore data for this Ligand-Target Pair
TargetDeoxyuridine 5'-triphosphate nucleotidohydrolase(Plasmodium falciparum)
Cardiff University
Curated by ChEMBL
Cardiff University
Curated by ChEMBL
Affinity DataKi: 200nMAssay Description:Inhibitory constant against Plasmodium falciparum deoxyuridine 5'-triphosphate nucleotidohydrolase expressed in Escherichia coli BL21 (DE3) cellsMore data for this Ligand-Target Pair
TargetDeoxyuridine 5'-triphosphate nucleotidohydrolase(Plasmodium falciparum)
Cardiff University
Curated by ChEMBL
Cardiff University
Curated by ChEMBL
Affinity DataKi: 240nMAssay Description:Inhibition of Plasmodium falciparum dUTPaseMore data for this Ligand-Target Pair
Affinity DataKi: 470nMAssay Description:Inhibitory kinetic constant against Dipeptidyl peptidase IV purified from CD26-negative C8166 cellsMore data for this Ligand-Target Pair
TargetDeoxyuridine 5'-triphosphate nucleotidohydrolase(Plasmodium falciparum)
Cardiff University
Curated by ChEMBL
Cardiff University
Curated by ChEMBL
Affinity DataKi: 600nMAssay Description:Inhibition of Plasmodium falciparum dUTPaseMore data for this Ligand-Target Pair
TargetDeoxyuridine 5'-triphosphate nucleotidohydrolase(Plasmodium falciparum)
Cardiff University
Curated by ChEMBL
Cardiff University
Curated by ChEMBL
Affinity DataKi: 700nMAssay Description:Inhibition of Plasmodium falciparum dUTPaseMore data for this Ligand-Target Pair
TargetDeoxyuridine 5'-triphosphate nucleotidohydrolase(Plasmodium falciparum)
Cardiff University
Curated by ChEMBL
Cardiff University
Curated by ChEMBL
Affinity DataKi: 900nMAssay Description:Inhibition of Plasmodium falciparum dUTPaseMore data for this Ligand-Target Pair
TargetDeoxyuridine 5'-triphosphate nucleotidohydrolase(Plasmodium falciparum)
Cardiff University
Curated by ChEMBL
Cardiff University
Curated by ChEMBL
Affinity DataKi: 900nMAssay Description:Inhibition of Plasmodium falciparum dUTPaseMore data for this Ligand-Target Pair
Affinity DataKi: 1.13E+3nM ΔG°: -35.3kJ/molepH: 4.5 T: 2°CAssay Description:Enzyme activities were assayed by monitoring the hydrolysis of substrate in the presence or absence of inhibitor compounds. The hydrolysis was record...More data for this Ligand-Target Pair
TargetDeoxyuridine 5'-triphosphate nucleotidohydrolase(Plasmodium falciparum)
Cardiff University
Curated by ChEMBL
Cardiff University
Curated by ChEMBL
Affinity DataKi: 1.16E+3nM ΔG°: -33.9kJ/molepH: 8.0 T: 2°CAssay Description:Nucleotide hydrolysis was monitored by mixing enzyme and substrate with a rapid kinetic accessory (Hi-Tech Scientific) attached to a spectrophotomete...More data for this Ligand-Target Pair
TargetDeoxyuridine 5'-triphosphate nucleotidohydrolase(Plasmodium falciparum)
Cardiff University
Curated by ChEMBL
Cardiff University
Curated by ChEMBL
Affinity DataKi: 1.20E+3nMAssay Description:Inhibition of Plasmodium falciparum dUTPaseMore data for this Ligand-Target Pair
TargetDeoxyuridine 5'-triphosphate nucleotidohydrolase(Plasmodium falciparum)
Cardiff University
Curated by ChEMBL
Cardiff University
Curated by ChEMBL
Affinity DataKi: 1.20E+3nMAssay Description:Inhibitory constant against Plasmodium falciparum deoxyuridine 5'-triphosphate nucleotidohydrolase expressed in Escherichia coli BL21 (DE3) cellsMore data for this Ligand-Target Pair
TargetDeoxyuridine 5'-triphosphate nucleotidohydrolase(Plasmodium falciparum)
Cardiff University
Curated by ChEMBL
Cardiff University
Curated by ChEMBL
Affinity DataKi: 1.30E+3nMAssay Description:Inhibitory constant against Plasmodium falciparum deoxyuridine 5'-triphosphate nucleotidohydrolase expressed in Escherichia coli BL21 (DE3) cellsMore data for this Ligand-Target Pair
TargetDeoxyuridine 5'-triphosphate nucleotidohydrolase(Plasmodium falciparum)
Cardiff University
Curated by ChEMBL
Cardiff University
Curated by ChEMBL
Affinity DataKi: 1.30E+3nMAssay Description:Inhibition of Plasmodium falciparum dUTPaseMore data for this Ligand-Target Pair
TargetDeoxyuridine 5'-triphosphate nucleotidohydrolase, mitochondrial(Homo sapiens (Human))
Cardiff University
Curated by ChEMBL
Cardiff University
Curated by ChEMBL
Affinity DataKi: 1.40E+3nMAssay Description:Inhibition of human recombinant dUTPaseMore data for this Ligand-Target Pair
TargetDeoxyuridine 5'-triphosphate nucleotidohydrolase, mitochondrial(Homo sapiens (Human))
Cardiff University
Curated by ChEMBL
Cardiff University
Curated by ChEMBL
Affinity DataKi: 1.40E+3nMAssay Description:Inhibition of human dUTPase using dUTP as substrate by spectrophotometric analysisMore data for this Ligand-Target Pair
TargetDeoxyuridine 5'-triphosphate nucleotidohydrolase(Plasmodium falciparum)
Cardiff University
Curated by ChEMBL
Cardiff University
Curated by ChEMBL
Affinity DataKi: 1.60E+3nMAssay Description:Inhibition of Plasmodium falciparum dUTPaseMore data for this Ligand-Target Pair
TargetDeoxyuridine 5'-triphosphate nucleotidohydrolase(Plasmodium falciparum)
Cardiff University
Curated by ChEMBL
Cardiff University
Curated by ChEMBL
Affinity DataKi: 1.60E+3nMAssay Description:Inhibition of Plasmodium falciparum dUTPaseMore data for this Ligand-Target Pair
TargetDeoxyuridine 5'-triphosphate nucleotidohydrolase(Plasmodium falciparum)
Cardiff University
Curated by ChEMBL
Cardiff University
Curated by ChEMBL
Affinity DataKi: 1.70E+3nMAssay Description:Inhibition of Plasmodium falciparum dUTPaseMore data for this Ligand-Target Pair
TargetDeoxyuridine 5'-triphosphate nucleotidohydrolase(Plasmodium falciparum)
Cardiff University
Curated by ChEMBL
Cardiff University
Curated by ChEMBL
Affinity DataKi: 1.80E+3nMAssay Description:Inhibition of Plasmodium falciparum dUTPaseMore data for this Ligand-Target Pair
TargetDeoxyuridine 5'-triphosphate nucleotidohydrolase(Plasmodium falciparum)
Cardiff University
Curated by ChEMBL
Cardiff University
Curated by ChEMBL
Affinity DataKi: 1.80E+3nMAssay Description:Inhibition of Plasmodium falciparum dUTPaseMore data for this Ligand-Target Pair
TargetDeoxyuridine 5'-triphosphate nucleotidohydrolase(Plasmodium falciparum)
Cardiff University
Curated by ChEMBL
Cardiff University
Curated by ChEMBL
Affinity DataKi: 1.80E+3nMAssay Description:Inhibitory constant against Plasmodium falciparum deoxyuridine 5'-triphosphate nucleotidohydrolase expressed in Escherichia coli BL21 (DE3) cellsMore data for this Ligand-Target Pair
TargetDeoxyuridine 5'-triphosphate nucleotidohydrolase(Plasmodium falciparum)
Cardiff University
Curated by ChEMBL
Cardiff University
Curated by ChEMBL
Affinity DataKi: 1.90E+3nM ΔG°: -32.7kJ/molepH: 8.0 T: 2°CAssay Description:Nucleotide hydrolysis was monitored by mixing enzyme and substrate with a rapid kinetic accessory (Hi-Tech Scientific) attached to a spectrophotomete...More data for this Ligand-Target Pair
TargetDeoxyuridine 5'-triphosphate nucleotidohydrolase(Plasmodium falciparum)
Cardiff University
Curated by ChEMBL
Cardiff University
Curated by ChEMBL
Affinity DataKi: 1.90E+3nMAssay Description:Inhibitory constant against Plasmodium falciparum deoxyuridine 5'-triphosphate nucleotidohydrolase expressed in Escherichia coli BL21 (DE3) cellsMore data for this Ligand-Target Pair
TargetDeoxyuridine 5'-triphosphate nucleotidohydrolase(Plasmodium falciparum)
Cardiff University
Curated by ChEMBL
Cardiff University
Curated by ChEMBL
Affinity DataKi: 2.00E+3nMAssay Description:Inhibition of Plasmodium falciparum dUTPaseMore data for this Ligand-Target Pair
TargetDeoxyuridine 5'-triphosphate nucleotidohydrolase(Plasmodium falciparum)
Cardiff University
Curated by ChEMBL
Cardiff University
Curated by ChEMBL
Affinity DataKi: 2.20E+3nMAssay Description:Inhibition of Plasmodium falciparum dUTPaseMore data for this Ligand-Target Pair
TargetDeoxyuridine 5'-triphosphate nucleotidohydrolase(Plasmodium falciparum)
Cardiff University
Curated by ChEMBL
Cardiff University
Curated by ChEMBL
Affinity DataKi: 2.20E+3nMAssay Description:Inhibition of Plasmodium falciparum dUTPaseMore data for this Ligand-Target Pair
TargetDeoxyuridine 5'-triphosphate nucleotidohydrolase(Plasmodium falciparum)
Cardiff University
Curated by ChEMBL
Cardiff University
Curated by ChEMBL
Affinity DataKi: 2.30E+3nMAssay Description:Inhibition of Plasmodium falciparum dUTPaseMore data for this Ligand-Target Pair
TargetDeoxyuridine 5'-triphosphate nucleotidohydrolase(Plasmodium falciparum)
Cardiff University
Curated by ChEMBL
Cardiff University
Curated by ChEMBL
Affinity DataKi: 2.50E+3nMAssay Description:Inhibition of Plasmodium falciparum dUTPaseMore data for this Ligand-Target Pair
TargetDeoxyuridine 5'-triphosphate nucleotidohydrolase(Plasmodium falciparum)
Cardiff University
Curated by ChEMBL
Cardiff University
Curated by ChEMBL
Affinity DataKi: 2.80E+3nMAssay Description:Inhibitory constant against Plasmodium falciparum deoxyuridine 5'-triphosphate nucleotidohydrolase expressed in Escherichia coli BL21 (DE3) cellsMore data for this Ligand-Target Pair
TargetDeoxyuridine 5'-triphosphate nucleotidohydrolase(Plasmodium falciparum)
Cardiff University
Curated by ChEMBL
Cardiff University
Curated by ChEMBL
Affinity DataKi: 2.80E+3nMAssay Description:Inhibition of Plasmodium falciparum dUTPaseMore data for this Ligand-Target Pair
TargetDeoxyuridine 5'-triphosphate nucleotidohydrolase(Plasmodium falciparum)
Cardiff University
Curated by ChEMBL
Cardiff University
Curated by ChEMBL
Affinity DataKi: 2.80E+3nM ΔG°: -31.7kJ/molepH: 8.0 T: 2°CAssay Description:Nucleotide hydrolysis was monitored by mixing enzyme and substrate with a rapid kinetic accessory (Hi-Tech Scientific) attached to a spectrophotomete...More data for this Ligand-Target Pair
Affinity DataKi: 3.13E+3nM ΔG°: -32.7kJ/molepH: 4.5 T: 2°CAssay Description:Enzyme activities were assayed by monitoring the hydrolysis of substrate in the presence or absence of inhibitor compounds. The hydrolysis was record...More data for this Ligand-Target Pair
TargetDeoxyuridine 5'-triphosphate nucleotidohydrolase(Plasmodium falciparum)
Cardiff University
Curated by ChEMBL
Cardiff University
Curated by ChEMBL
Affinity DataKi: 3.80E+3nMAssay Description:Inhibition of Plasmodium falciparum dUTPaseMore data for this Ligand-Target Pair
TargetDeoxyuridine 5'-triphosphate nucleotidohydrolase(Plasmodium falciparum)
Cardiff University
Curated by ChEMBL
Cardiff University
Curated by ChEMBL
Affinity DataKi: 4.20E+3nMAssay Description:Inhibitory constant against Plasmodium falciparum deoxyuridine 5'-triphosphate nucleotidohydrolase expressed in Escherichia coli BL21 (DE3) cellsMore data for this Ligand-Target Pair
TargetDeoxyuridine 5'-triphosphate nucleotidohydrolase(Plasmodium falciparum)
Cardiff University
Curated by ChEMBL
Cardiff University
Curated by ChEMBL
Affinity DataKi: 4.20E+3nM ΔG°: -30.7kJ/molepH: 8.0 T: 2°CAssay Description:Nucleotide hydrolysis was monitored by mixing enzyme and substrate with a rapid kinetic accessory (Hi-Tech Scientific) attached to a spectrophotomete...More data for this Ligand-Target Pair
TargetDeoxyuridine 5'-triphosphate nucleotidohydrolase(Plasmodium falciparum)
Cardiff University
Curated by ChEMBL
Cardiff University
Curated by ChEMBL
Affinity DataKi: 4.30E+3nMAssay Description:Inhibition of Plasmodium falciparum dUTPaseMore data for this Ligand-Target Pair



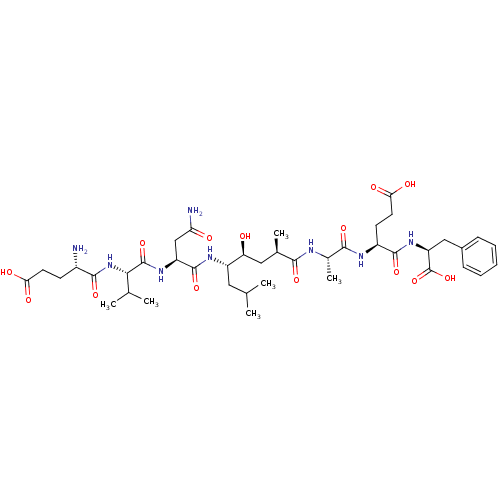
 3D Structure (crystal)
3D Structure (crystal)