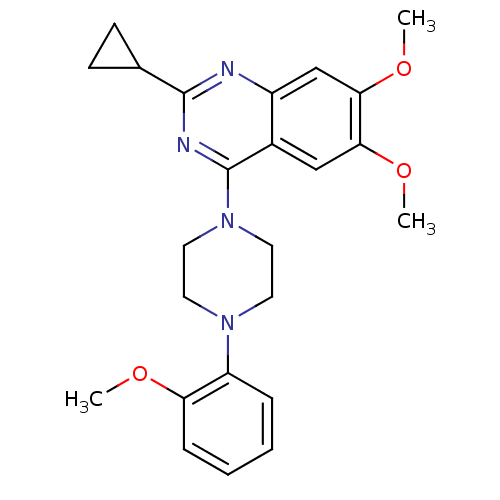
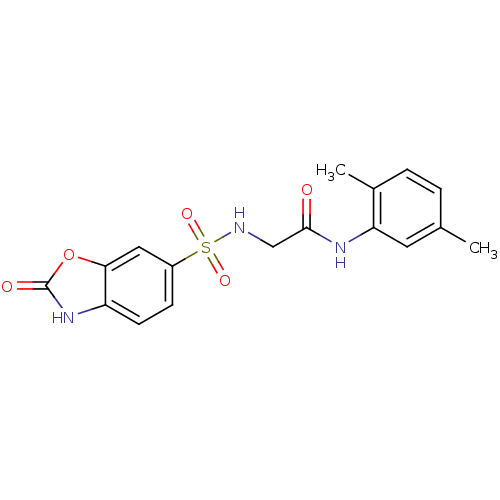
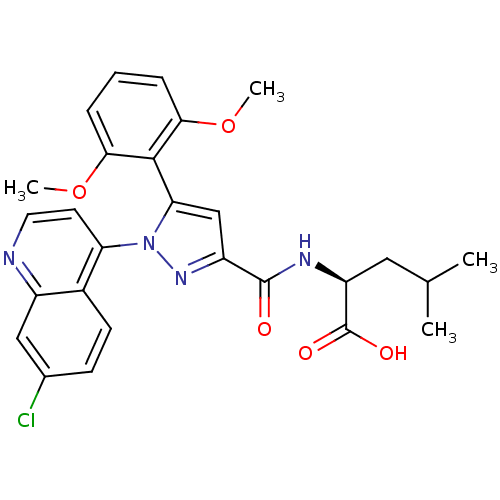
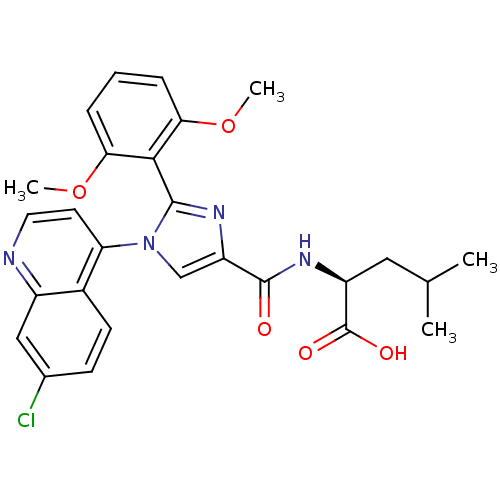
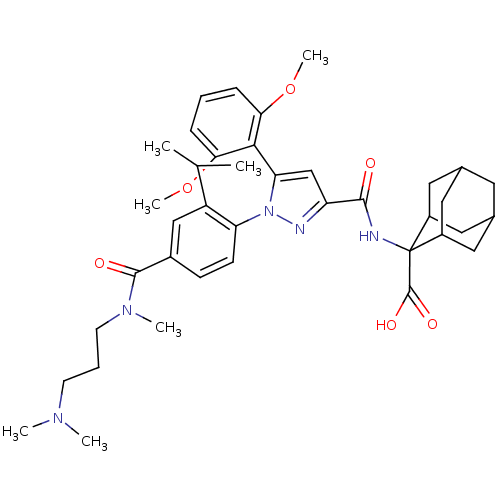
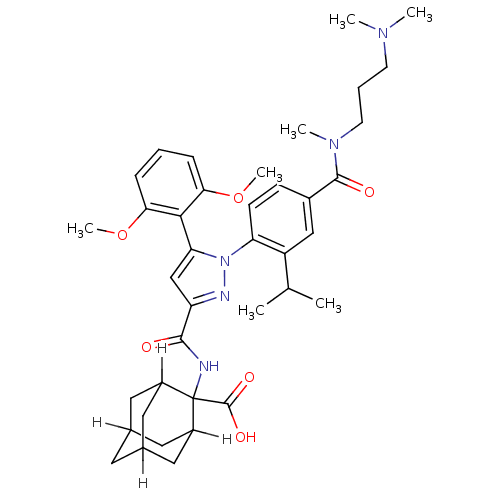
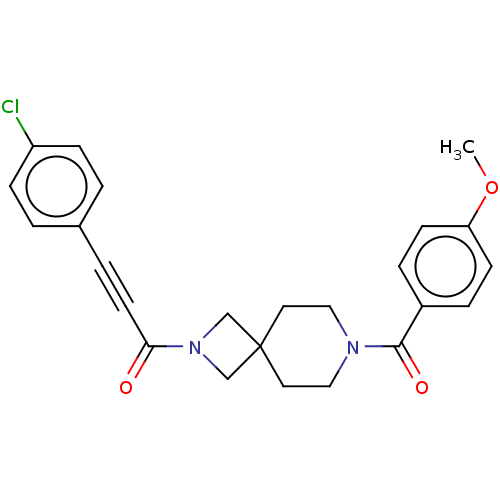
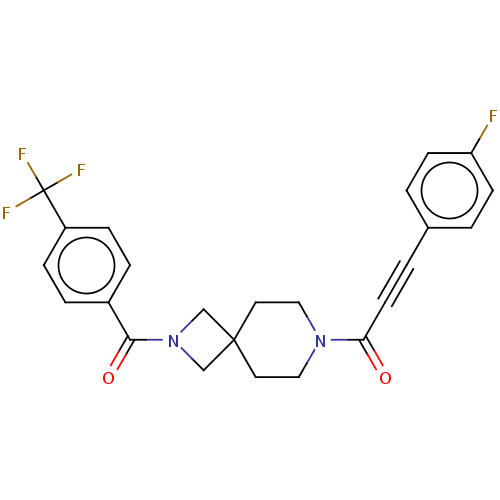
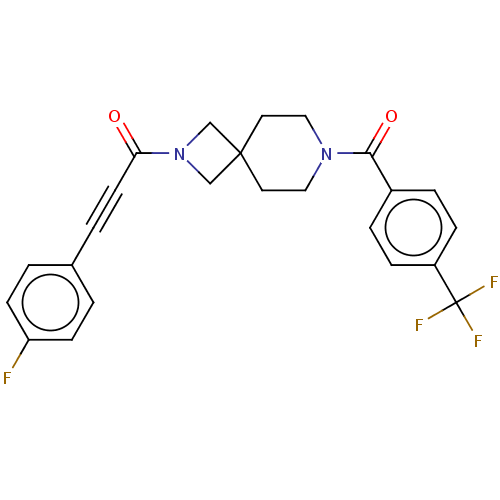
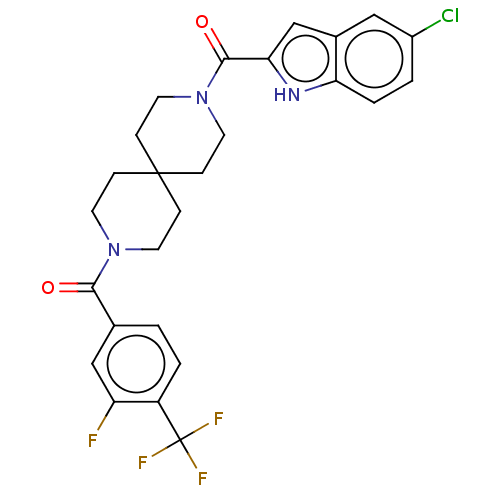
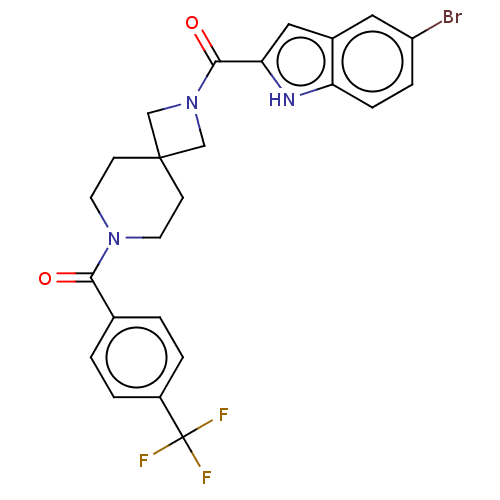
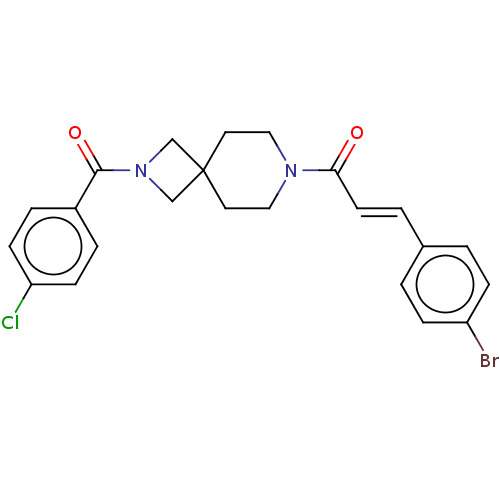
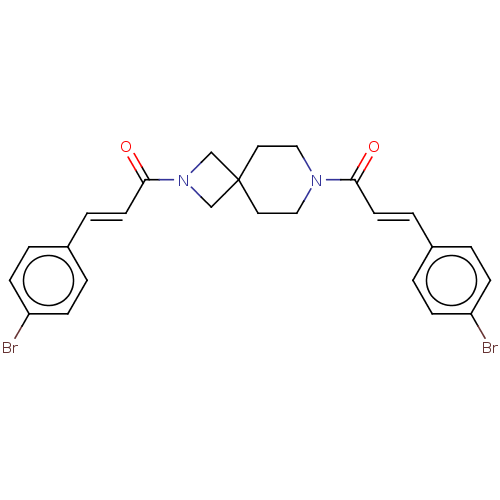
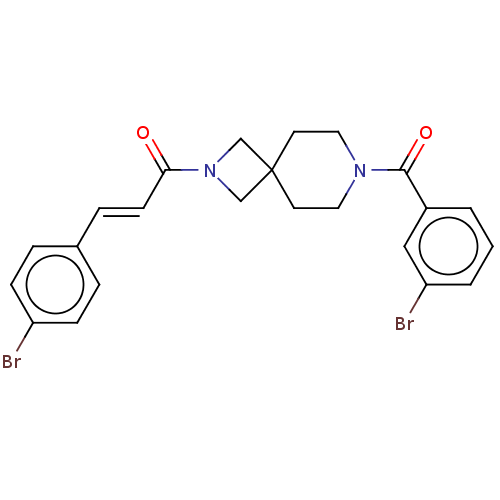
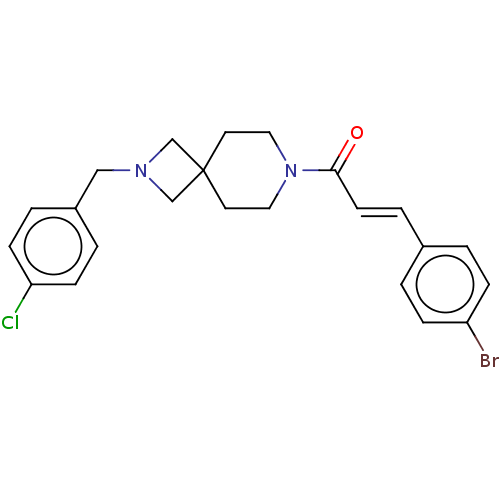
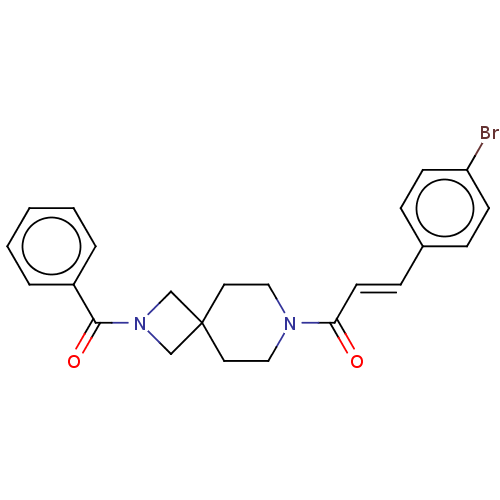
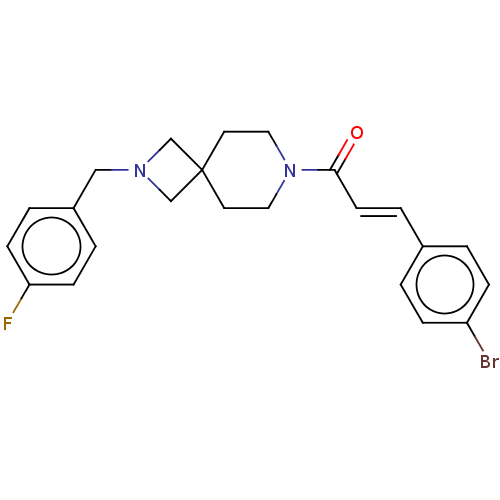
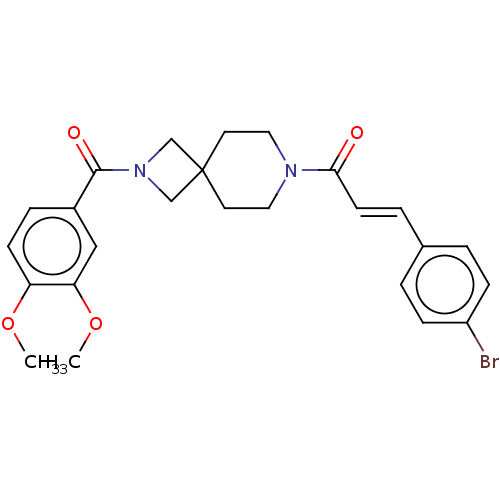
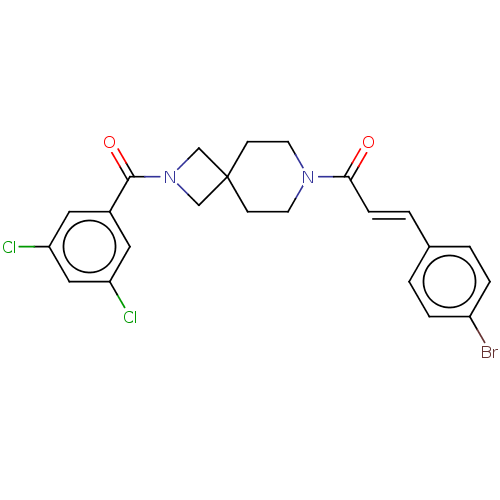
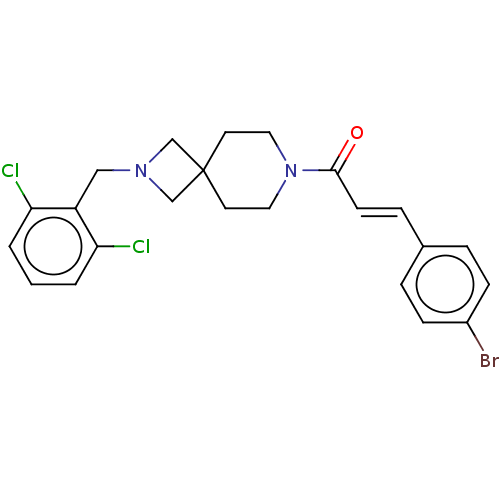
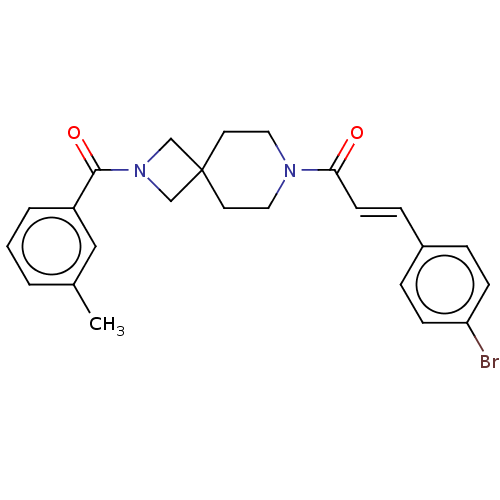
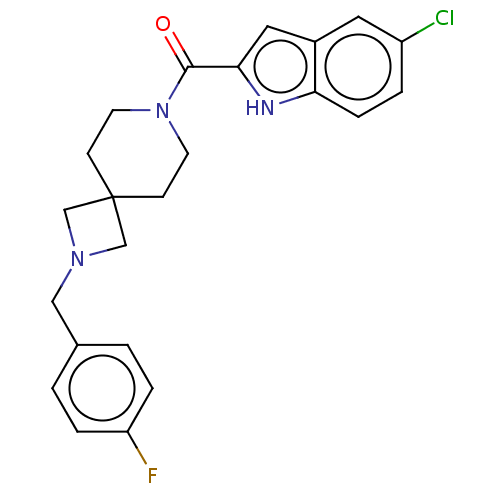
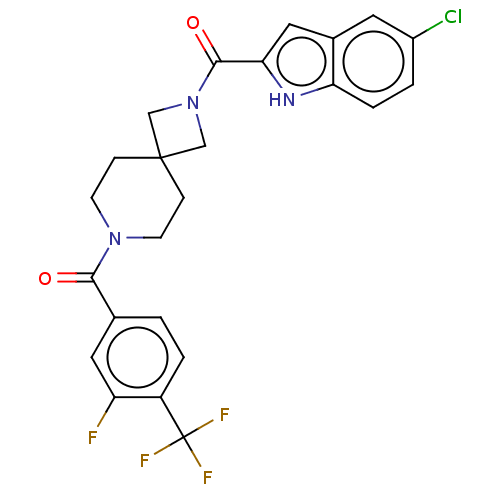
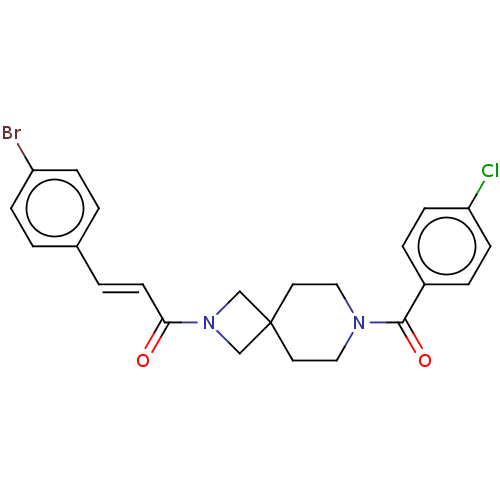
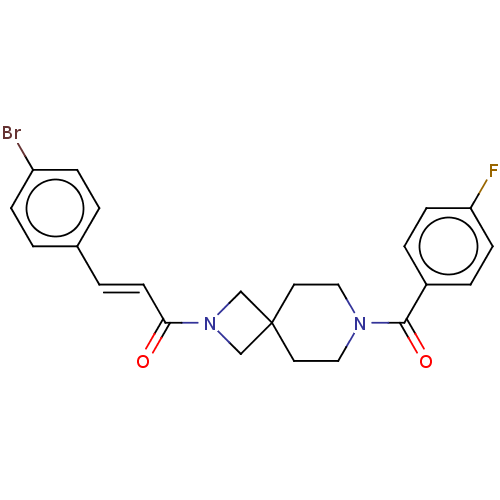
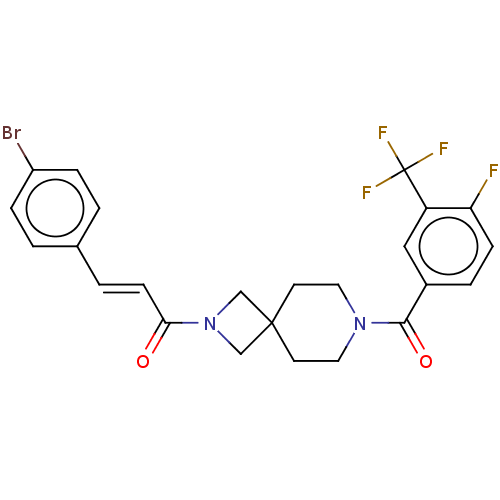
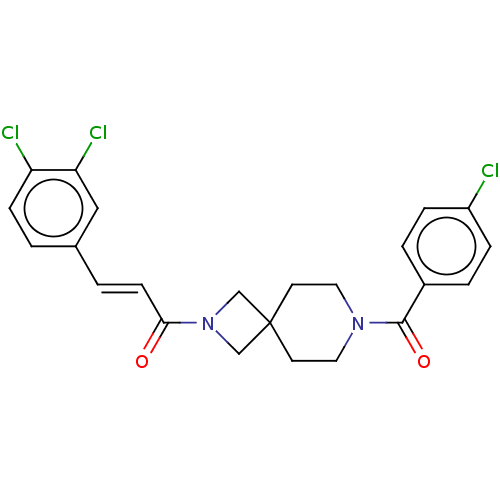
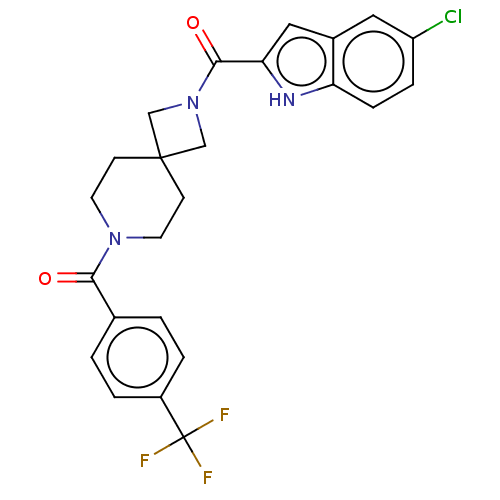
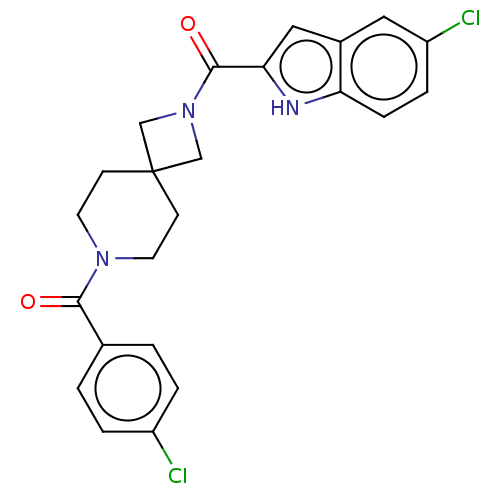
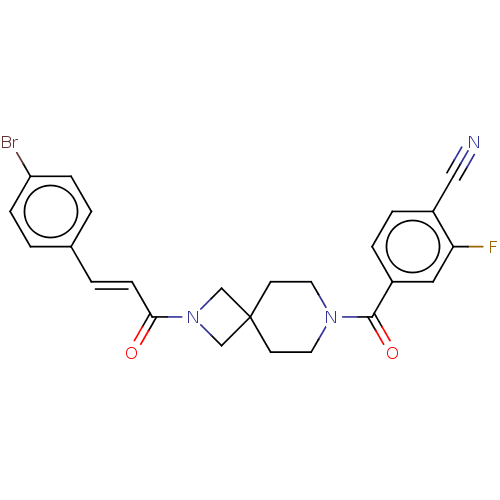
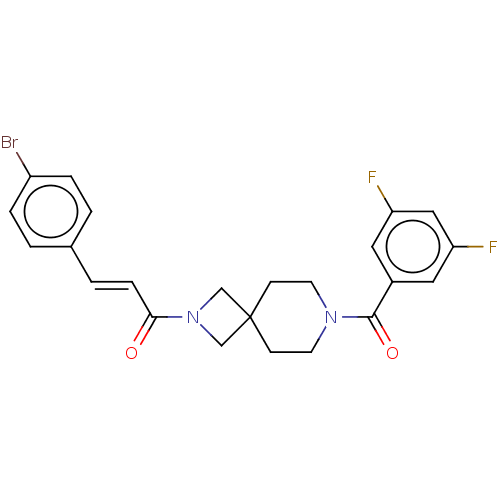
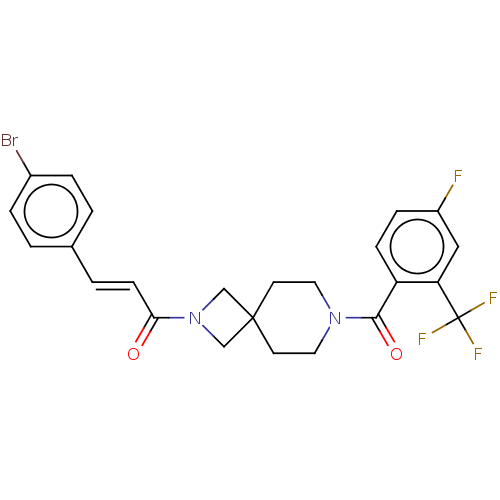
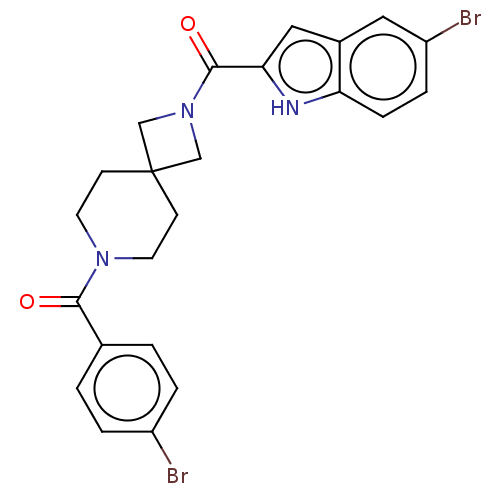
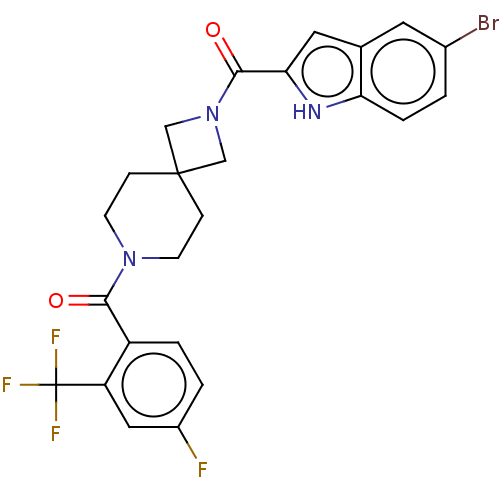
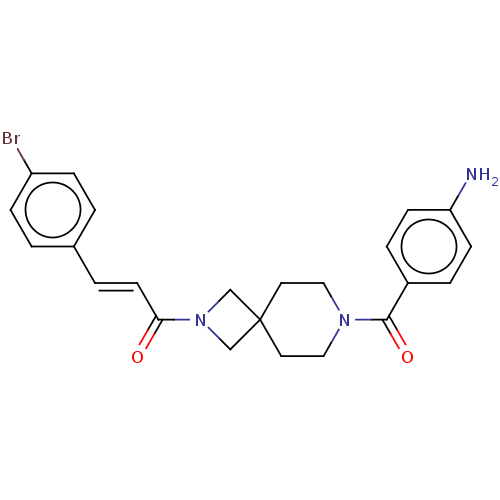
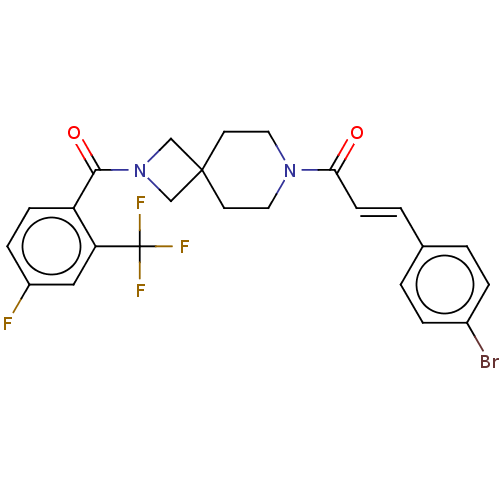
TargetSigma non-opioid intracellular receptor 1(Homo sapiens (Human))
Sanford-Burnham Medical Research Institute
Curated by ChEMBL
Sanford-Burnham Medical Research Institute
Curated by ChEMBL
Affinity DataKi: 410nMAssay Description:Binding affinity to sigma-1 receptor (unknown origin)More data for this Ligand-Target Pair
Target5-hydroxytryptamine receptor 3A(Homo sapiens (Human))
Sanford-Burnham Medical Research Institute
Curated by ChEMBL
Sanford-Burnham Medical Research Institute
Curated by ChEMBL
Affinity DataKi: 1.00E+3nMAssay Description:Binding affinity to 5-HT3 receptor (unknown origin)More data for this Ligand-Target Pair
TargetHistamine H1 receptor(Homo sapiens (Human))
Sanford-Burnham Medical Research Institute
Curated by ChEMBL
Sanford-Burnham Medical Research Institute
Curated by ChEMBL
Affinity DataKi: 1.42E+3nMAssay Description:Binding affinity to histamine H1 receptor (unknown origin)More data for this Ligand-Target Pair
TargetTranslocator protein(Homo sapiens (Human))
Sanford-Burnham Medical Research Institute
Curated by ChEMBL
Sanford-Burnham Medical Research Institute
Curated by ChEMBL
Affinity DataKi: 2.54E+3nMAssay Description:Binding affinity to PBR receptor (unknown origin)More data for this Ligand-Target Pair
TargetIntestinal-type alkaline phosphatase(Mus musculus)
Sanford-Burnham Medical Research Institute
Curated by ChEMBL
Sanford-Burnham Medical Research Institute
Curated by ChEMBL
Affinity DataKi: 3.20E+3nMAssay Description:Competitive inhibition of mouse duodenal-specific FLAG-tagged IAP expressed in African green monkey COS1 cells using p-nitrophenyl phosphate as subst...More data for this Ligand-Target Pair
TargetNeurotensin receptor type 1(Homo sapiens (Human))
Sanford-Burnham Medical Research Institute
Curated by ChEMBL
Sanford-Burnham Medical Research Institute
Curated by ChEMBL
Affinity DataKi: 3.30E+3nMAssay Description:Inhibition of NTS1 receptor (unknown origin)More data for this Ligand-Target Pair
TargetMu-type opioid receptor(Homo sapiens (Human))
Sanford-Burnham Medical Research Institute
Curated by ChEMBL
Sanford-Burnham Medical Research Institute
Curated by ChEMBL
Affinity DataKi: 3.40E+3nMAssay Description:Inhibition of MOR (unknown origin)More data for this Ligand-Target Pair
TargetDelta-type opioid receptor(Homo sapiens (Human))
Sanford-Burnham Medical Research Institute
Curated by ChEMBL
Sanford-Burnham Medical Research Institute
Curated by ChEMBL
Affinity DataKi: 5.20E+3nMAssay Description:Inhibition of DOR (unknown origin)More data for this Ligand-Target Pair
TargetSodium-dependent dopamine transporter(Homo sapiens (Human))
Sanford-Burnham Medical Research Institute
Curated by ChEMBL
Sanford-Burnham Medical Research Institute
Curated by ChEMBL
Affinity DataKi: >1.00E+4nMAssay Description:Inhibition of DAT (unknown origin)More data for this Ligand-Target Pair
TargetNeurotensin receptor type 1(Homo sapiens (Human))
Sanford-Burnham Medical Research Institute
Curated by ChEMBL
Sanford-Burnham Medical Research Institute
Curated by ChEMBL
Affinity DataKi: 1.00E+4nMAssay Description:Inhibition of NTS1 receptor (unknown origin)More data for this Ligand-Target Pair
TargetSodium-dependent dopamine transporter(Homo sapiens (Human))
Sanford-Burnham Medical Research Institute
Curated by ChEMBL
Sanford-Burnham Medical Research Institute
Curated by ChEMBL
Affinity DataKi: >1.00E+4nMAssay Description:Inhibition of DAT (unknown origin)More data for this Ligand-Target Pair
TargetNeurotensin receptor type 1(Homo sapiens (Human))
Sanford-Burnham Medical Research Institute
Curated by ChEMBL
Sanford-Burnham Medical Research Institute
Curated by ChEMBL
Affinity DataIC50: 0.240nMAssay Description:Displacement of [125I]-neurotensin from NTR1 (unknown origin)More data for this Ligand-Target Pair
TargetNeurotensin receptor type 1(Homo sapiens (Human))
Sanford-Burnham Medical Research Institute
Curated by ChEMBL
Sanford-Burnham Medical Research Institute
Curated by ChEMBL
Affinity DataIC50: 0.240nMAssay Description:Displacement of [125I]-neurotensin from NTR1 in HUVEC after 1 hr by gamma counting analysisMore data for this Ligand-Target Pair
TargetNeurotensin receptor type 1(Homo sapiens (Human))
Sanford-Burnham Medical Research Institute
Curated by ChEMBL
Sanford-Burnham Medical Research Institute
Curated by ChEMBL
Affinity DataIC50: 4nMAssay Description:Antagonist activity at NTR1 (unknown origin)More data for this Ligand-Target Pair
TargetG-protein coupled receptor 183(Homo sapiens (Human))
Sanford Burnham Prebys Medical Discovery Institute
US Patent
Sanford Burnham Prebys Medical Discovery Institute
US Patent
Affinity DataIC50: <5nMAssay Description:The test compounds that demonstrated a corrected % activity of >=50% were defined as inhibitors of the reaction. The experimental values were normali...More data for this Ligand-Target Pair
TargetG-protein coupled receptor 183(Homo sapiens (Human))
Sanford Burnham Prebys Medical Discovery Institute
US Patent
Sanford Burnham Prebys Medical Discovery Institute
US Patent
Affinity DataIC50: <5nMAssay Description:The test compounds that demonstrated a corrected % activity of >=50% were defined as inhibitors of the reaction. The experimental values were normali...More data for this Ligand-Target Pair
TargetG-protein coupled receptor 183(Homo sapiens (Human))
Sanford Burnham Prebys Medical Discovery Institute
US Patent
Sanford Burnham Prebys Medical Discovery Institute
US Patent
Affinity DataIC50: <5nMAssay Description:The test compounds that demonstrated a corrected % activity of >=50% were defined as inhibitors of the reaction. The experimental values were normali...More data for this Ligand-Target Pair
TargetG-protein coupled receptor 183(Homo sapiens (Human))
Sanford Burnham Prebys Medical Discovery Institute
US Patent
Sanford Burnham Prebys Medical Discovery Institute
US Patent
Affinity DataIC50: <5nMAssay Description:The test compounds that demonstrated a corrected % activity of >=50% were defined as inhibitors of the reaction. The experimental values were normali...More data for this Ligand-Target Pair
TargetG-protein coupled receptor 183(Homo sapiens (Human))
Sanford Burnham Prebys Medical Discovery Institute
US Patent
Sanford Burnham Prebys Medical Discovery Institute
US Patent
Affinity DataIC50: <5nMAssay Description:The test compounds that demonstrated a corrected % activity of >=50% were defined as inhibitors of the reaction. The experimental values were normali...More data for this Ligand-Target Pair
TargetG-protein coupled receptor 183(Homo sapiens (Human))
Sanford Burnham Prebys Medical Discovery Institute
US Patent
Sanford Burnham Prebys Medical Discovery Institute
US Patent
Affinity DataIC50: <5nMAssay Description:The test compounds that demonstrated a corrected % activity of >=50% were defined as inhibitors of the reaction. The experimental values were normali...More data for this Ligand-Target Pair
TargetG-protein coupled receptor 183(Homo sapiens (Human))
Sanford Burnham Prebys Medical Discovery Institute
US Patent
Sanford Burnham Prebys Medical Discovery Institute
US Patent
Affinity DataIC50: <5nMAssay Description:The test compounds that demonstrated a corrected % activity of >=50% were defined as inhibitors of the reaction. The experimental values were normali...More data for this Ligand-Target Pair
TargetG-protein coupled receptor 183(Homo sapiens (Human))
Sanford Burnham Prebys Medical Discovery Institute
US Patent
Sanford Burnham Prebys Medical Discovery Institute
US Patent
Affinity DataIC50: <5nMAssay Description:The test compounds that demonstrated a corrected % activity of >=50% were defined as inhibitors of the reaction. The experimental values were normali...More data for this Ligand-Target Pair
TargetG-protein coupled receptor 183(Homo sapiens (Human))
Sanford Burnham Prebys Medical Discovery Institute
US Patent
Sanford Burnham Prebys Medical Discovery Institute
US Patent
Affinity DataIC50: <5nMAssay Description:The test compounds that demonstrated a corrected % activity of >=50% were defined as inhibitors of the reaction. The experimental values were normali...More data for this Ligand-Target Pair
TargetG-protein coupled receptor 183(Homo sapiens (Human))
Sanford Burnham Prebys Medical Discovery Institute
US Patent
Sanford Burnham Prebys Medical Discovery Institute
US Patent
Affinity DataIC50: <5nMAssay Description:The test compounds that demonstrated a corrected % activity of >=50% were defined as inhibitors of the reaction. The experimental values were normali...More data for this Ligand-Target Pair
TargetG-protein coupled receptor 183(Homo sapiens (Human))
Sanford Burnham Prebys Medical Discovery Institute
US Patent
Sanford Burnham Prebys Medical Discovery Institute
US Patent
Affinity DataIC50: <5nMAssay Description:The test compounds that demonstrated a corrected % activity of >=50% were defined as inhibitors of the reaction. The experimental values were normali...More data for this Ligand-Target Pair
TargetG-protein coupled receptor 183(Homo sapiens (Human))
Sanford Burnham Prebys Medical Discovery Institute
US Patent
Sanford Burnham Prebys Medical Discovery Institute
US Patent
Affinity DataIC50: <5nMAssay Description:The test compounds that demonstrated a corrected % activity of >=50% were defined as inhibitors of the reaction. The experimental values were normali...More data for this Ligand-Target Pair
TargetG-protein coupled receptor 183(Homo sapiens (Human))
Sanford Burnham Prebys Medical Discovery Institute
US Patent
Sanford Burnham Prebys Medical Discovery Institute
US Patent
Affinity DataIC50: <5nMAssay Description:The test compounds that demonstrated a corrected % activity of >=50% were defined as inhibitors of the reaction. The experimental values were normali...More data for this Ligand-Target Pair
TargetG-protein coupled receptor 183(Homo sapiens (Human))
Sanford Burnham Prebys Medical Discovery Institute
US Patent
Sanford Burnham Prebys Medical Discovery Institute
US Patent
Affinity DataIC50: <5nMAssay Description:The test compounds that demonstrated a corrected % activity of >=50% were defined as inhibitors of the reaction. The experimental values were normali...More data for this Ligand-Target Pair
TargetG-protein coupled receptor 183(Homo sapiens (Human))
Sanford Burnham Prebys Medical Discovery Institute
US Patent
Sanford Burnham Prebys Medical Discovery Institute
US Patent
Affinity DataIC50: <5nMAssay Description:The test compounds that demonstrated a corrected % activity of >=50% were defined as inhibitors of the reaction. The experimental values were normali...More data for this Ligand-Target Pair
TargetG-protein coupled receptor 183(Homo sapiens (Human))
Sanford Burnham Prebys Medical Discovery Institute
US Patent
Sanford Burnham Prebys Medical Discovery Institute
US Patent
Affinity DataIC50: <5nMAssay Description:The test compounds that demonstrated a corrected % activity of >=50% were defined as inhibitors of the reaction. The experimental values were normali...More data for this Ligand-Target Pair
TargetG-protein coupled receptor 183(Homo sapiens (Human))
Sanford Burnham Prebys Medical Discovery Institute
US Patent
Sanford Burnham Prebys Medical Discovery Institute
US Patent
Affinity DataIC50: <5nMAssay Description:The test compounds that demonstrated a corrected % activity of >=50% were defined as inhibitors of the reaction. The experimental values were normali...More data for this Ligand-Target Pair
TargetG-protein coupled receptor 183(Homo sapiens (Human))
Sanford Burnham Prebys Medical Discovery Institute
US Patent
Sanford Burnham Prebys Medical Discovery Institute
US Patent
Affinity DataIC50: <5nMAssay Description:The test compounds that demonstrated a corrected % activity of >=50% were defined as inhibitors of the reaction. The experimental values were normali...More data for this Ligand-Target Pair
TargetG-protein coupled receptor 183(Homo sapiens (Human))
Sanford Burnham Prebys Medical Discovery Institute
US Patent
Sanford Burnham Prebys Medical Discovery Institute
US Patent
Affinity DataIC50: <5nMAssay Description:The test compounds that demonstrated a corrected % activity of >=50% were defined as inhibitors of the reaction. The experimental values were normali...More data for this Ligand-Target Pair
TargetG-protein coupled receptor 183(Homo sapiens (Human))
Sanford Burnham Prebys Medical Discovery Institute
US Patent
Sanford Burnham Prebys Medical Discovery Institute
US Patent
Affinity DataIC50: <5nMAssay Description:The test compounds that demonstrated a corrected % activity of >=50% were defined as inhibitors of the reaction. The experimental values were normali...More data for this Ligand-Target Pair
TargetG-protein coupled receptor 183(Homo sapiens (Human))
Sanford Burnham Prebys Medical Discovery Institute
US Patent
Sanford Burnham Prebys Medical Discovery Institute
US Patent
Affinity DataIC50: <5nMAssay Description:The test compounds that demonstrated a corrected % activity of >=50% were defined as inhibitors of the reaction. The experimental values were normali...More data for this Ligand-Target Pair
TargetG-protein coupled receptor 183(Homo sapiens (Human))
Sanford Burnham Prebys Medical Discovery Institute
US Patent
Sanford Burnham Prebys Medical Discovery Institute
US Patent
Affinity DataIC50: <5nMAssay Description:The test compounds that demonstrated a corrected % activity of >=50% were defined as inhibitors of the reaction. The experimental values were normali...More data for this Ligand-Target Pair
TargetG-protein coupled receptor 183(Homo sapiens (Human))
Sanford Burnham Prebys Medical Discovery Institute
US Patent
Sanford Burnham Prebys Medical Discovery Institute
US Patent
Affinity DataIC50: <5nMAssay Description:The test compounds that demonstrated a corrected % activity of >=50% were defined as inhibitors of the reaction. The experimental values were normali...More data for this Ligand-Target Pair
TargetG-protein coupled receptor 183(Homo sapiens (Human))
Sanford Burnham Prebys Medical Discovery Institute
US Patent
Sanford Burnham Prebys Medical Discovery Institute
US Patent
Affinity DataIC50: <5nMAssay Description:The test compounds that demonstrated a corrected % activity of >=50% were defined as inhibitors of the reaction. The experimental values were normali...More data for this Ligand-Target Pair
TargetG-protein coupled receptor 183(Homo sapiens (Human))
Sanford Burnham Prebys Medical Discovery Institute
US Patent
Sanford Burnham Prebys Medical Discovery Institute
US Patent
Affinity DataIC50: <5nMAssay Description:The test compounds that demonstrated a corrected % activity of >=50% were defined as inhibitors of the reaction. The experimental values were normali...More data for this Ligand-Target Pair
TargetG-protein coupled receptor 183(Homo sapiens (Human))
Sanford Burnham Prebys Medical Discovery Institute
US Patent
Sanford Burnham Prebys Medical Discovery Institute
US Patent
Affinity DataIC50: <5nMAssay Description:The test compounds that demonstrated a corrected % activity of >=50% were defined as inhibitors of the reaction. The experimental values were normali...More data for this Ligand-Target Pair
TargetG-protein coupled receptor 183(Homo sapiens (Human))
Sanford Burnham Prebys Medical Discovery Institute
US Patent
Sanford Burnham Prebys Medical Discovery Institute
US Patent
Affinity DataIC50: <5nMAssay Description:The test compounds that demonstrated a corrected % activity of >=50% were defined as inhibitors of the reaction. The experimental values were normali...More data for this Ligand-Target Pair
TargetG-protein coupled receptor 183(Homo sapiens (Human))
Sanford Burnham Prebys Medical Discovery Institute
US Patent
Sanford Burnham Prebys Medical Discovery Institute
US Patent
Affinity DataIC50: <5nMAssay Description:The test compounds that demonstrated a corrected % activity of >=50% were defined as inhibitors of the reaction. The experimental values were normali...More data for this Ligand-Target Pair
TargetG-protein coupled receptor 183(Homo sapiens (Human))
Sanford Burnham Prebys Medical Discovery Institute
US Patent
Sanford Burnham Prebys Medical Discovery Institute
US Patent
Affinity DataIC50: <5nMAssay Description:The test compounds that demonstrated a corrected % activity of >=50% were defined as inhibitors of the reaction. The experimental values were normali...More data for this Ligand-Target Pair
TargetG-protein coupled receptor 183(Homo sapiens (Human))
Sanford Burnham Prebys Medical Discovery Institute
US Patent
Sanford Burnham Prebys Medical Discovery Institute
US Patent
Affinity DataIC50: <5nMAssay Description:The test compounds that demonstrated a corrected % activity of >=50% were defined as inhibitors of the reaction. The experimental values were normali...More data for this Ligand-Target Pair
TargetG-protein coupled receptor 183(Homo sapiens (Human))
Sanford Burnham Prebys Medical Discovery Institute
US Patent
Sanford Burnham Prebys Medical Discovery Institute
US Patent
Affinity DataIC50: <5nMAssay Description:The test compounds that demonstrated a corrected % activity of >=50% were defined as inhibitors of the reaction. The experimental values were normali...More data for this Ligand-Target Pair
TargetG-protein coupled receptor 183(Homo sapiens (Human))
Sanford Burnham Prebys Medical Discovery Institute
US Patent
Sanford Burnham Prebys Medical Discovery Institute
US Patent
Affinity DataIC50: <5nMAssay Description:The test compounds that demonstrated a corrected % activity of >=50% were defined as inhibitors of the reaction. The experimental values were normali...More data for this Ligand-Target Pair
TargetG-protein coupled receptor 183(Homo sapiens (Human))
Sanford Burnham Prebys Medical Discovery Institute
US Patent
Sanford Burnham Prebys Medical Discovery Institute
US Patent
Affinity DataIC50: <5nMAssay Description:The test compounds that demonstrated a corrected % activity of >=50% were defined as inhibitors of the reaction. The experimental values were normali...More data for this Ligand-Target Pair
TargetG-protein coupled receptor 183(Homo sapiens (Human))
Sanford Burnham Prebys Medical Discovery Institute
US Patent
Sanford Burnham Prebys Medical Discovery Institute
US Patent
Affinity DataIC50: <5nMAssay Description:The test compounds that demonstrated a corrected % activity of >=50% were defined as inhibitors of the reaction. The experimental values were normali...More data for this Ligand-Target Pair
TargetG-protein coupled receptor 183(Homo sapiens (Human))
Sanford Burnham Prebys Medical Discovery Institute
US Patent
Sanford Burnham Prebys Medical Discovery Institute
US Patent
Affinity DataIC50: <5nMAssay Description:The test compounds that demonstrated a corrected % activity of >=50% were defined as inhibitors of the reaction. The experimental values were normali...More data for this Ligand-Target Pair
TargetG-protein coupled receptor 183(Homo sapiens (Human))
Sanford Burnham Prebys Medical Discovery Institute
US Patent
Sanford Burnham Prebys Medical Discovery Institute
US Patent
Affinity DataIC50: <5nMAssay Description:The test compounds that demonstrated a corrected % activity of >=50% were defined as inhibitors of the reaction. The experimental values were normali...More data for this Ligand-Target Pair