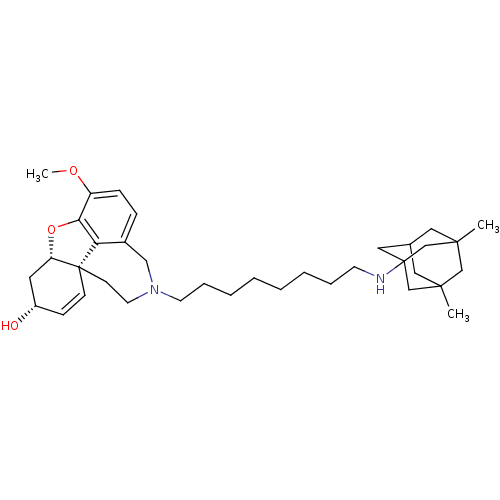
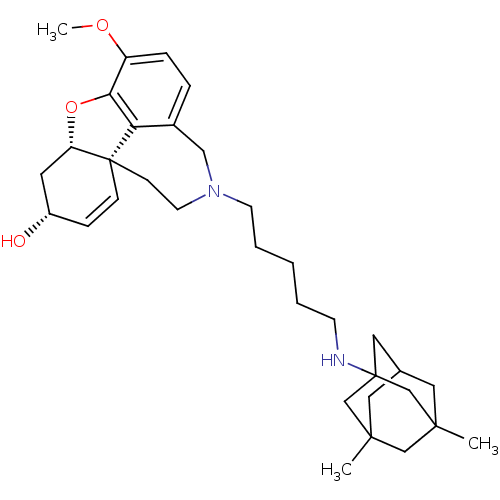
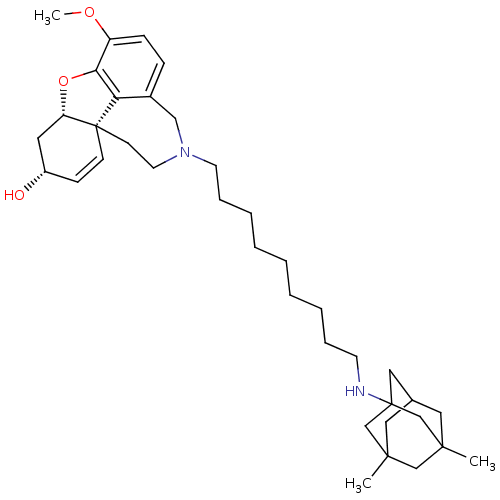
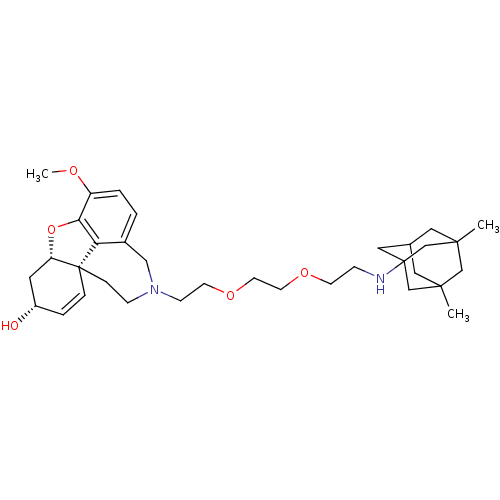
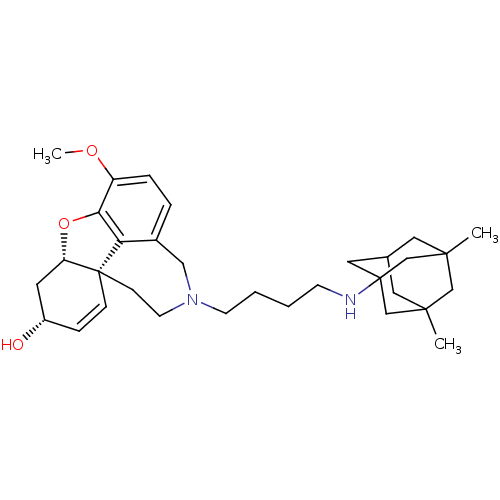
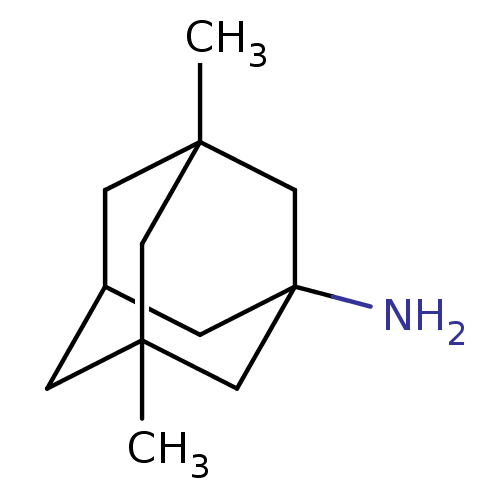
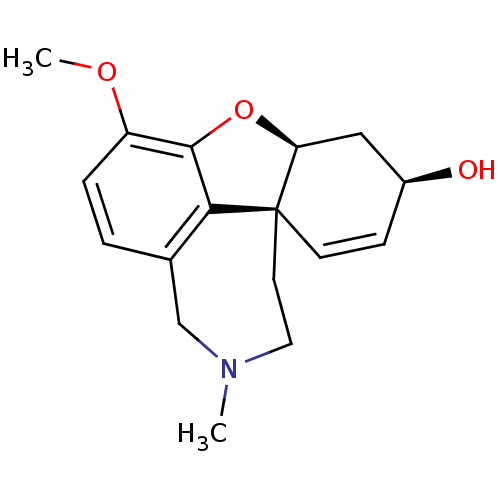
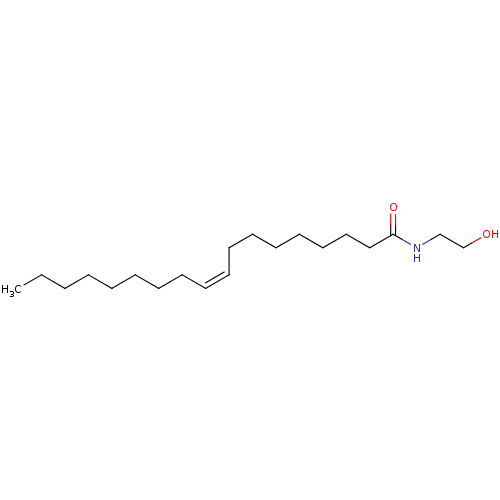
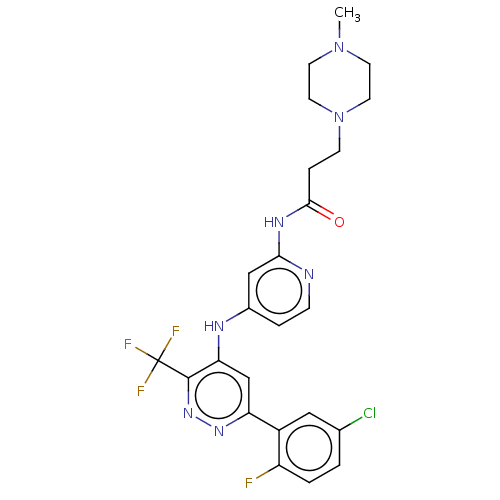
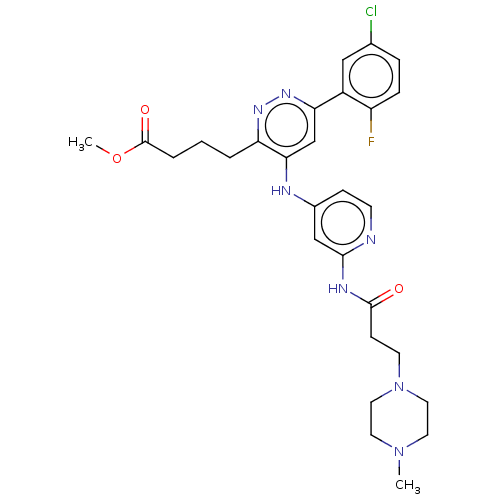
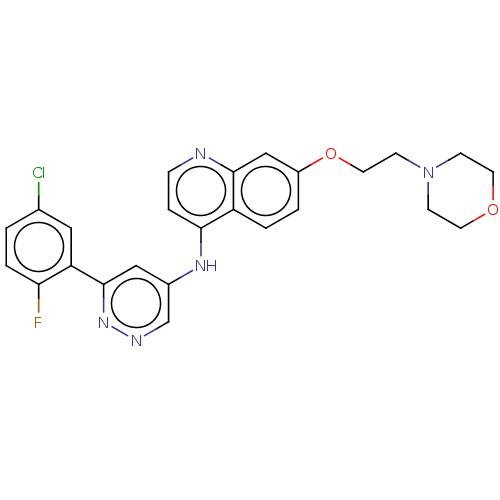
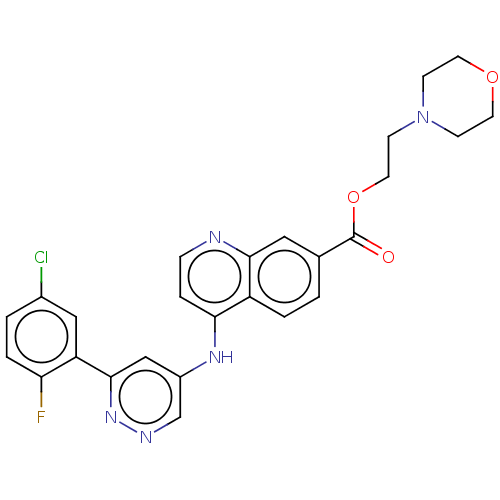
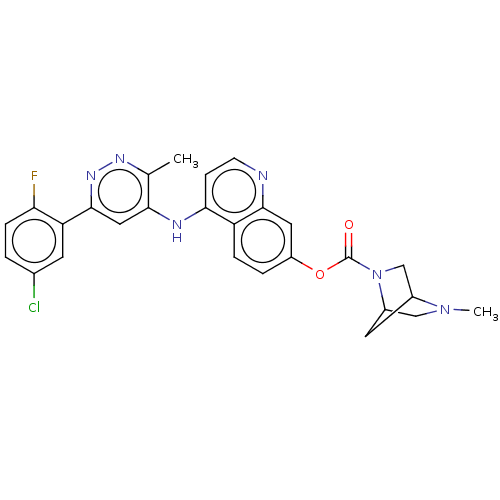
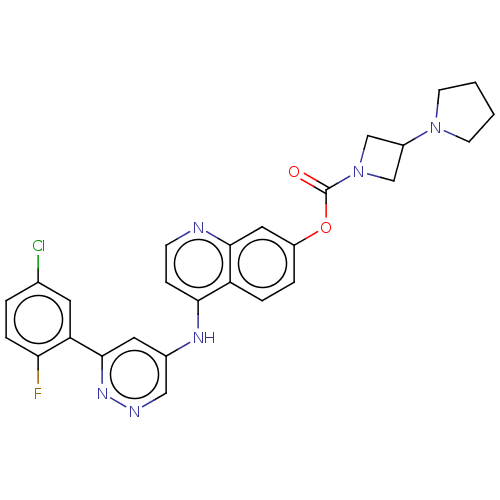
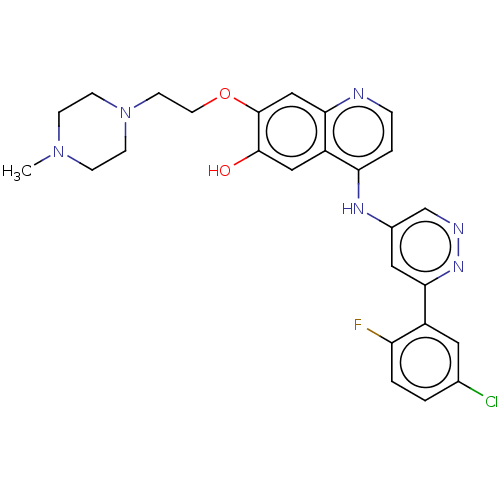
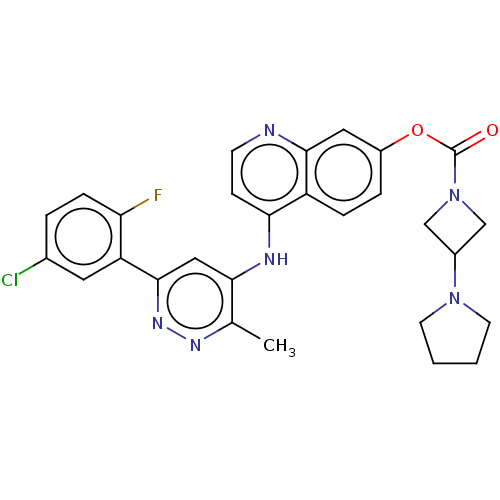
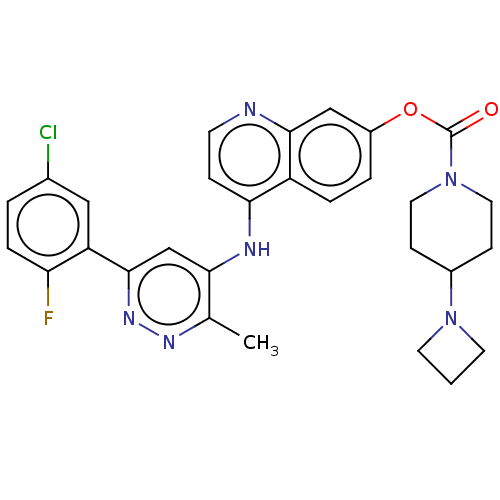
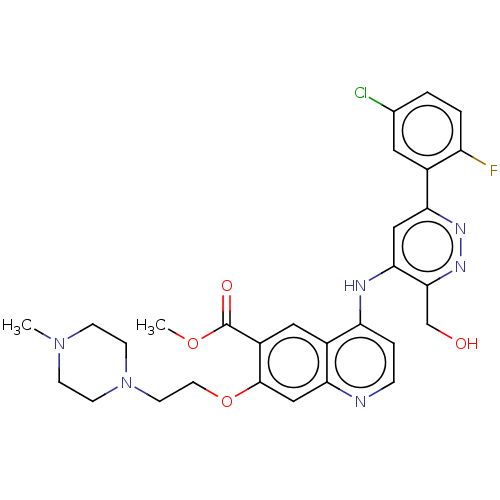
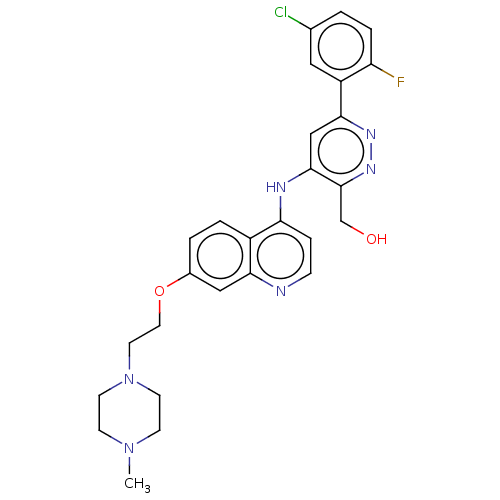
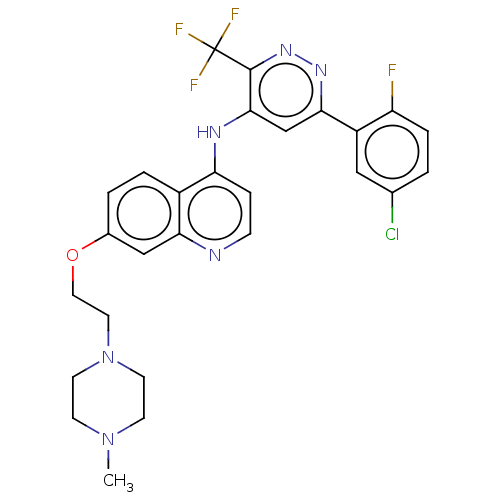
TargetGlutamate receptor ionotropic, NMDA 2B(Rattus norvegicus (Rat))
Istituto Italiano Di Tecnologia
Curated by ChEMBL
Istituto Italiano Di Tecnologia
Curated by ChEMBL
Affinity DataKi: 29nMAssay Description:Displacement of [3H]Ifenprodil from NMDAR-2B in Sprague-Dawley rat frontal cortex homogenates after 2 hrs by liquid scintillation countingMore data for this Ligand-Target Pair
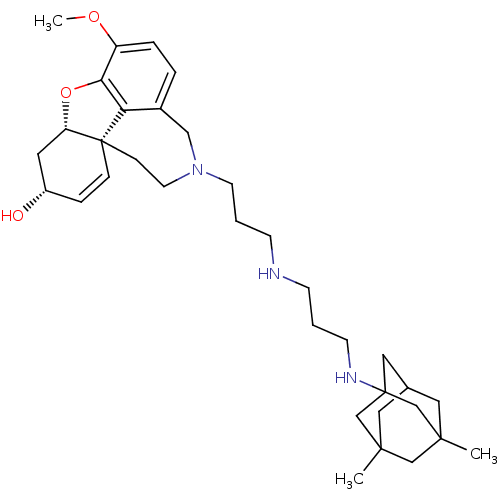
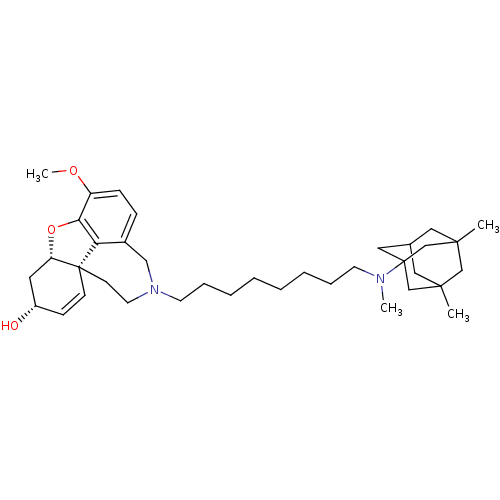
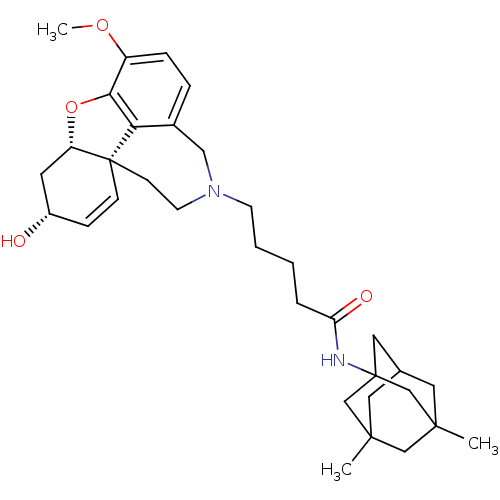
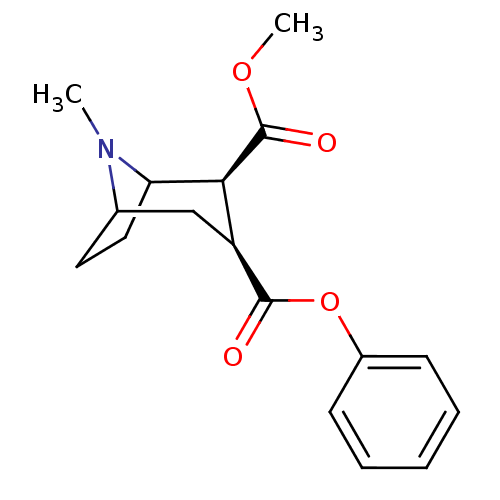
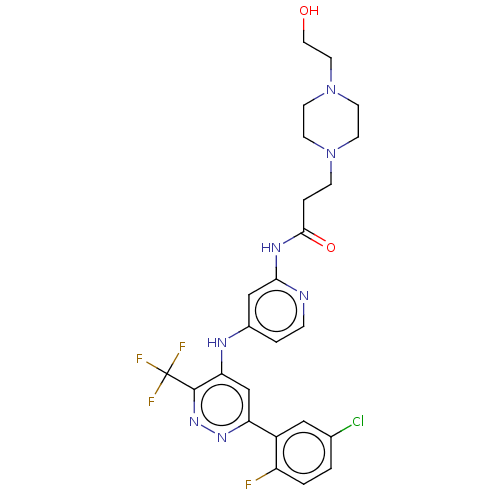
TargetGlutamate receptor ionotropic, NMDA 2B(Rattus norvegicus (Rat))
Istituto Italiano Di Tecnologia
Curated by ChEMBL
Istituto Italiano Di Tecnologia
Curated by ChEMBL
Affinity DataKi: 2.90E+3nMAssay Description:Displacement of [3H]Ifenprodil from NMDAR-2B in Sprague-Dawley rat frontal cortex homogenates after 2 hrs by liquid scintillation countingMore data for this Ligand-Target Pair
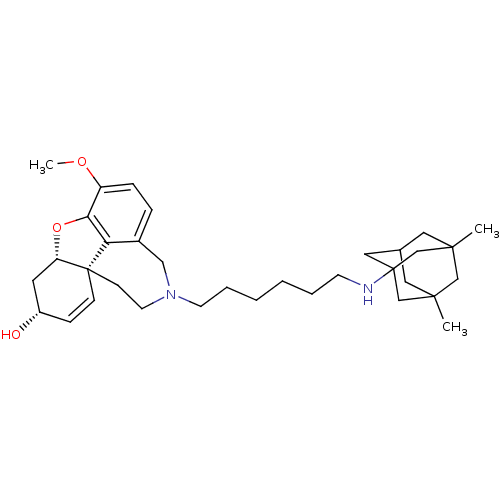
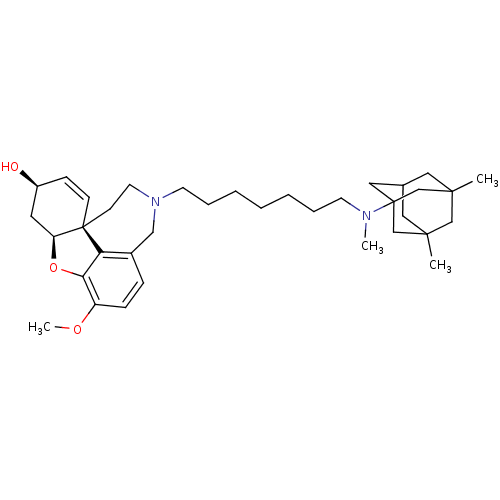
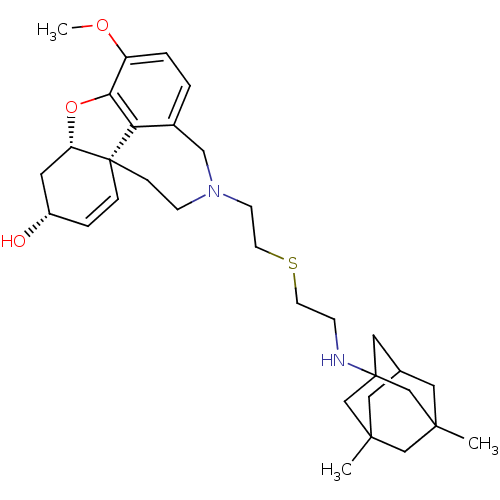
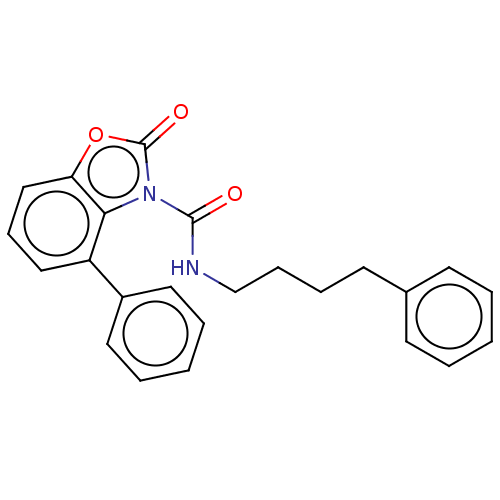
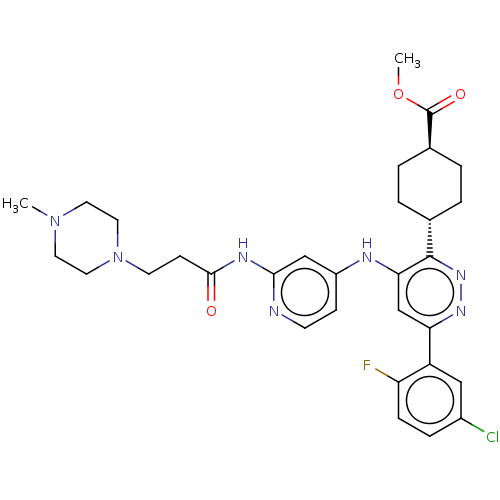
TargetGlutamate receptor ionotropic, NMDA 2B(Rattus norvegicus (Rat))
Istituto Italiano Di Tecnologia
Curated by ChEMBL
Istituto Italiano Di Tecnologia
Curated by ChEMBL
Affinity DataKi: 3.32E+3nMAssay Description:Displacement of [3H]Ifenprodil from NMDAR-2B in Sprague-Dawley rat frontal cortex homogenates after 2 hrs by liquid scintillation countingMore data for this Ligand-Target Pair
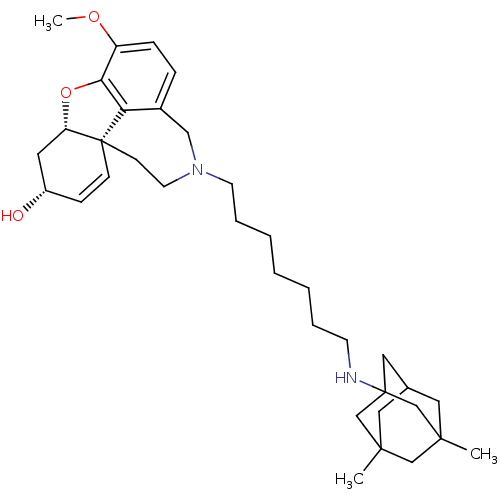
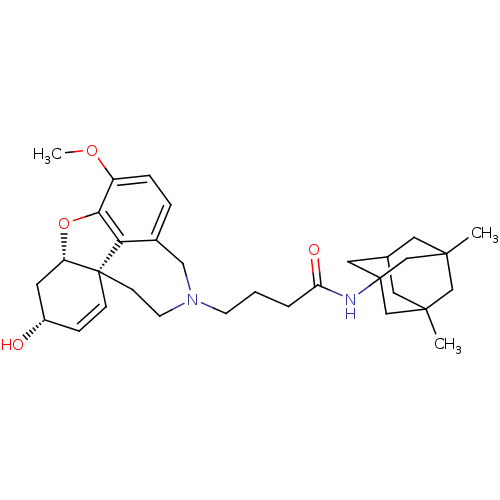
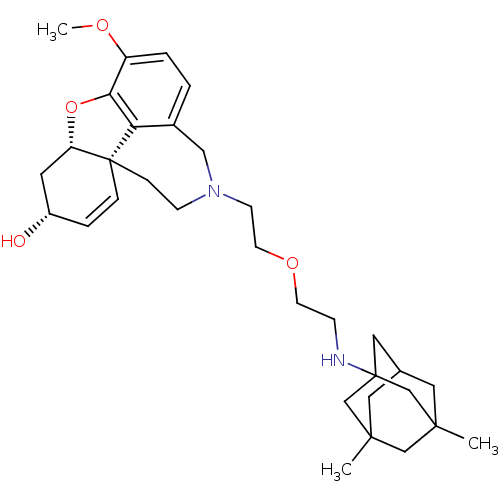
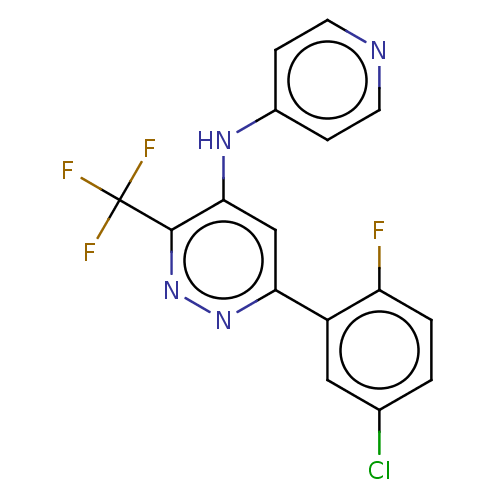
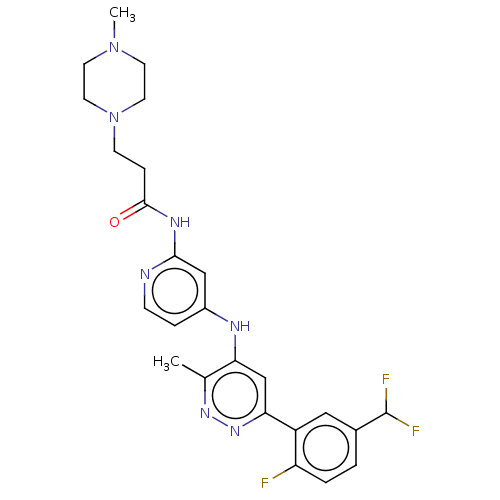
TargetGlutamate receptor ionotropic, NMDA 2B(Rattus norvegicus (Rat))
Istituto Italiano Di Tecnologia
Curated by ChEMBL
Istituto Italiano Di Tecnologia
Curated by ChEMBL
Affinity DataKi: 4.60E+3nMAssay Description:Displacement of [3H]Ifenprodil from NMDAR-2B in Sprague-Dawley rat frontal cortex homogenates after 2 hrs by liquid scintillation countingMore data for this Ligand-Target Pair
TargetGlutamate receptor ionotropic, NMDA 2B(Rattus norvegicus (Rat))
Istituto Italiano Di Tecnologia
Curated by ChEMBL
Istituto Italiano Di Tecnologia
Curated by ChEMBL
Affinity DataKi: 5.25E+3nMAssay Description:Displacement of [3H]Ifenprodil from NMDAR-2B in Sprague-Dawley rat frontal cortex homogenates after 2 hrs by liquid scintillation countingMore data for this Ligand-Target Pair
TargetGlutamate receptor ionotropic, NMDA 2B(Rattus norvegicus (Rat))
Istituto Italiano Di Tecnologia
Curated by ChEMBL
Istituto Italiano Di Tecnologia
Curated by ChEMBL
Affinity DataKi: 9.76E+3nMAssay Description:Displacement of [3H]Ifenprodil from NMDAR-2B in Sprague-Dawley rat frontal cortex homogenates after 2 hrs by liquid scintillation countingMore data for this Ligand-Target Pair
TargetGlutamate receptor ionotropic, NMDA 2B(Rattus norvegicus (Rat))
Istituto Italiano Di Tecnologia
Curated by ChEMBL
Istituto Italiano Di Tecnologia
Curated by ChEMBL
Affinity DataKi: 1.10E+4nMAssay Description:Displacement of [3H]Ifenprodil from NMDAR-2B in Sprague-Dawley rat frontal cortex homogenates after 2 hrs by liquid scintillation countingMore data for this Ligand-Target Pair
TargetGlutamate receptor ionotropic, NMDA 2B(Rattus norvegicus (Rat))
Istituto Italiano Di Tecnologia
Curated by ChEMBL
Istituto Italiano Di Tecnologia
Curated by ChEMBL
Affinity DataKi: 4.09E+4nMAssay Description:Displacement of [3H]Ifenprodil from NMDAR-2B in Sprague-Dawley rat frontal cortex homogenates after 2 hrs by liquid scintillation countingMore data for this Ligand-Target Pair
TargetGlutamate receptor ionotropic, NMDA 2B(Rattus norvegicus (Rat))
Istituto Italiano Di Tecnologia
Curated by ChEMBL
Istituto Italiano Di Tecnologia
Curated by ChEMBL
Affinity DataKi: >1.00E+5nMAssay Description:Displacement of [3H]Ifenprodil from NMDAR-2B in Sprague-Dawley rat frontal cortex homogenates after 2 hrs by liquid scintillation countingMore data for this Ligand-Target Pair
TargetGlutamate receptor ionotropic, NMDA 2B(Rattus norvegicus (Rat))
Istituto Italiano Di Tecnologia
Curated by ChEMBL
Istituto Italiano Di Tecnologia
Curated by ChEMBL
Affinity DataKi: >1.00E+5nMAssay Description:Displacement of [3H]Ifenprodil from NMDAR-2B in Sprague-Dawley rat frontal cortex homogenates after 2 hrs by liquid scintillation countingMore data for this Ligand-Target Pair
TargetGlutamate receptor ionotropic, NMDA 2B(Rattus norvegicus (Rat))
Istituto Italiano Di Tecnologia
Curated by ChEMBL
Istituto Italiano Di Tecnologia
Curated by ChEMBL
Affinity DataKi: >1.00E+5nMAssay Description:Displacement of [3H]Ifenprodil from NMDAR-2B in Sprague-Dawley rat frontal cortex homogenates after 2 hrs by liquid scintillation countingMore data for this Ligand-Target Pair
TargetGlutamate receptor ionotropic, NMDA 2B(Rattus norvegicus (Rat))
Istituto Italiano Di Tecnologia
Curated by ChEMBL
Istituto Italiano Di Tecnologia
Curated by ChEMBL
Affinity DataKi: >1.00E+5nMAssay Description:Displacement of [3H]Ifenprodil from NMDAR-2B in Sprague-Dawley rat frontal cortex homogenates after 2 hrs by liquid scintillation countingMore data for this Ligand-Target Pair
TargetGlutamate receptor ionotropic, NMDA 2B(Rattus norvegicus (Rat))
Istituto Italiano Di Tecnologia
Curated by ChEMBL
Istituto Italiano Di Tecnologia
Curated by ChEMBL
Affinity DataKi: >1.00E+5nMAssay Description:Displacement of [3H]Ifenprodil from NMDAR-2B in Sprague-Dawley rat frontal cortex homogenates after 2 hrs by liquid scintillation countingMore data for this Ligand-Target Pair
TargetGlutamate receptor ionotropic, NMDA 2B(Rattus norvegicus (Rat))
Istituto Italiano Di Tecnologia
Curated by ChEMBL
Istituto Italiano Di Tecnologia
Curated by ChEMBL
Affinity DataKi: >1.00E+5nMAssay Description:Displacement of [3H]Ifenprodil from NMDAR-2B in Sprague-Dawley rat frontal cortex homogenates after 2 hrs by liquid scintillation countingMore data for this Ligand-Target Pair
TargetGlutamate receptor ionotropic, NMDA 2B(Rattus norvegicus (Rat))
Istituto Italiano Di Tecnologia
Curated by ChEMBL
Istituto Italiano Di Tecnologia
Curated by ChEMBL
Affinity DataKi: >1.00E+5nMAssay Description:Displacement of [3H]Ifenprodil from NMDAR-2B in Sprague-Dawley rat frontal cortex homogenates after 2 hrs by liquid scintillation countingMore data for this Ligand-Target Pair
TargetGlutamate receptor ionotropic, NMDA 2B(Rattus norvegicus (Rat))
Istituto Italiano Di Tecnologia
Curated by ChEMBL
Istituto Italiano Di Tecnologia
Curated by ChEMBL
Affinity DataKi: >1.00E+5nMAssay Description:Displacement of [3H]Ifenprodil from NMDAR-2B in Sprague-Dawley rat frontal cortex homogenates after 2 hrs by liquid scintillation countingMore data for this Ligand-Target Pair
TargetGlutamate receptor ionotropic, NMDA 2B(Rattus norvegicus (Rat))
Istituto Italiano Di Tecnologia
Curated by ChEMBL
Istituto Italiano Di Tecnologia
Curated by ChEMBL
Affinity DataKi: >1.00E+5nMAssay Description:Displacement of [3H]Ifenprodil from NMDAR-2B in Sprague-Dawley rat frontal cortex homogenates after 2 hrs by liquid scintillation countingMore data for this Ligand-Target Pair
TargetGlutamate receptor ionotropic, NMDA 2B(Rattus norvegicus (Rat))
Istituto Italiano Di Tecnologia
Curated by ChEMBL
Istituto Italiano Di Tecnologia
Curated by ChEMBL
Affinity DataKi: >1.00E+5nMAssay Description:Displacement of [3H]Ifenprodil from NMDAR-2B in Sprague-Dawley rat frontal cortex homogenates after 2 hrs by liquid scintillation countingMore data for this Ligand-Target Pair
TargetGlutamate receptor ionotropic, NMDA 2B(Rattus norvegicus (Rat))
Istituto Italiano Di Tecnologia
Curated by ChEMBL
Istituto Italiano Di Tecnologia
Curated by ChEMBL
Affinity DataKi: >1.00E+5nMAssay Description:Displacement of [3H]Ifenprodil from NMDAR-2B in Sprague-Dawley rat frontal cortex homogenates after 2 hrs by liquid scintillation countingMore data for this Ligand-Target Pair
TargetGlutamate receptor ionotropic, NMDA 2B(Rattus norvegicus (Rat))
Istituto Italiano Di Tecnologia
Curated by ChEMBL
Istituto Italiano Di Tecnologia
Curated by ChEMBL
Affinity DataKi: >1.00E+5nMAssay Description:Displacement of [3H]Ifenprodil from NMDAR-2B in Sprague-Dawley rat frontal cortex homogenates after 2 hrs by liquid scintillation countingMore data for this Ligand-Target Pair
TargetAcid ceramidase(Homo sapiens (Human))
Fondazione Istituto Italiano Di Tecnologia
Curated by ChEMBL
Fondazione Istituto Italiano Di Tecnologia
Curated by ChEMBL
Affinity DataKi: 5.00E+5nMAssay Description:Inhibition of acid ceramidase (unknown origin)More data for this Ligand-Target Pair
Affinity DataIC50: 0.5nMAssay Description:Displacement of [3H]paroxetine from Serotonin transporter of rat caudate putamenMore data for this Ligand-Target Pair
Affinity DataIC50: 0.520nMAssay Description:Inhibition of AChE in Wistar rat brain homogenates using acetylthiocholine iodide and DTNB as substrate after 10 mins by Ellman methodMore data for this Ligand-Target Pair
TargetAcid ceramidase(Homo sapiens (Human))
Fondazione Istituto Italiano Di Tecnologia
Curated by ChEMBL
Fondazione Istituto Italiano Di Tecnologia
Curated by ChEMBL
Affinity DataIC50: 0.800nMAssay Description:The assay was performed in Optiplate 96-wells black plates, with each reaction well containing a mixture of 25 mM sodium acetate buffer pH 4.5 and a ...More data for this Ligand-Target Pair
TargetAcid ceramidase(Homo sapiens (Human))
Fondazione Istituto Italiano Di Tecnologia
Curated by ChEMBL
Fondazione Istituto Italiano Di Tecnologia
Curated by ChEMBL
Affinity DataIC50: 0.800nMAssay Description:Inhibition of recombinant human acid ceramidase expressed in HEK293 cells using N-[(1S,2R)-2-hydroxy-1-(hydroxymethyl)-4-(2-oxochromen-7-yl)-oxybutyl...More data for this Ligand-Target Pair
Affinity DataIC50: <1nMAssay Description:The enzymatic activity of compounds of the present invention was monitored measuring the formation of ADP using the ADP-GLO Kinases assay. Following ...More data for this Ligand-Target Pair
Ligand Info
Affinity DataIC50: <1nMAssay Description:The enzymatic activity of compounds of the present invention was monitored measuring the formation of ADP using the ADP-GLO Kinases assay. Following ...More data for this Ligand-Target Pair
Ligand Info
Affinity DataIC50: <1nMAssay Description:The enzymatic activity of compounds of the present invention was monitored measuring the formation of ADP using the ADP-GLO Kinases assay. Following ...More data for this Ligand-Target Pair
Ligand Info
Affinity DataIC50: <1nMAssay Description:The enzymatic activity of compounds of the present invention was monitored measuring the formation of ADP using the ADP-GLO Kinases assay. Following ...More data for this Ligand-Target Pair
Ligand Info
Affinity DataIC50: <1nMAssay Description:The enzymatic activity of compounds of the present invention was monitored measuring the formation of ADP using the ADP-GLO Kinases assay. Following ...More data for this Ligand-Target Pair
Ligand Info
Affinity DataIC50: <1nMAssay Description:The enzymatic activity of compounds of the present invention was monitored measuring the formation of ADP using the ADP-GLO Kinases assay. Following ...More data for this Ligand-Target Pair
Ligand Info
Affinity DataIC50: <1nMAssay Description:The enzymatic activity of compounds of the present invention was monitored measuring the formation of ADP using the ADP-GLO Kinases assay. Following ...More data for this Ligand-Target Pair
Ligand Info
Affinity DataIC50: <1nMAssay Description:The enzymatic activity of compounds of the present invention was monitored measuring the formation of ADP using the ADP-GLO Kinases assay. Following ...More data for this Ligand-Target Pair
Ligand Info
Affinity DataIC50: <1nMAssay Description:The enzymatic activity of compounds of the present invention was monitored measuring the formation of ADP using the ADP-GLO Kinases assay. Following ...More data for this Ligand-Target Pair
Ligand Info
Affinity DataIC50: <1nMAssay Description:The enzymatic activity of compounds of the present invention was monitored measuring the formation of ADP using the ADP-GLO Kinases assay. Following ...More data for this Ligand-Target Pair
Ligand Info
Affinity DataIC50: <1nMAssay Description:The enzymatic activity of compounds of the present invention was monitored measuring the formation of ADP using the ADP-GLO Kinases assay. Following ...More data for this Ligand-Target Pair
Ligand Info
Affinity DataIC50: <1nMAssay Description:The enzymatic activity of compounds of the present invention was monitored measuring the formation of ADP using the ADP-GLO Kinases assay. Following ...More data for this Ligand-Target Pair
Ligand Info
Affinity DataIC50: <1nMAssay Description:The enzymatic activity of compounds of the present invention was monitored measuring the formation of ADP using the ADP-GLO Kinases assay. Following ...More data for this Ligand-Target Pair
Ligand Info
Affinity DataIC50: <1nMAssay Description:The enzymatic activity of compounds of the present invention was monitored measuring the formation of ADP using the ADP-GLO Kinases assay. Following ...More data for this Ligand-Target Pair
Ligand Info
Affinity DataIC50: <1nMAssay Description:The enzymatic activity of compounds of the present invention was monitored measuring the formation of ADP using the ADP-GLO Kinases assay. Following ...More data for this Ligand-Target Pair
Ligand Info
Affinity DataIC50: <1nMAssay Description:The enzymatic activity of compounds of the present invention was monitored measuring the formation of ADP using the ADP-GLO Kinases assay. Following ...More data for this Ligand-Target Pair
Ligand Info
Affinity DataIC50: <1nMAssay Description:The enzymatic activity of compounds of the present invention was monitored measuring the formation of ADP using the ADP-GLO Kinases assay. Following ...More data for this Ligand-Target Pair
Ligand Info
Affinity DataIC50: <1nMAssay Description:The enzymatic activity of compounds of the present invention was monitored measuring the formation of ADP using the ADP-GLO Kinases assay. Following ...More data for this Ligand-Target Pair
Ligand Info
Affinity DataIC50: <1nMAssay Description:The enzymatic activity of compounds of the present invention was monitored measuring the formation of ADP using the ADP-GLO Kinases assay. Following ...More data for this Ligand-Target Pair
Ligand Info
Affinity DataIC50: <1nMAssay Description:The enzymatic activity of compounds of the present invention was monitored measuring the formation of ADP using the ADP-GLO Kinases assay. Following ...More data for this Ligand-Target Pair
Ligand Info
Affinity DataIC50: <1nMAssay Description:The enzymatic activity of compounds of the present invention was monitored measuring the formation of ADP using the ADP-GLO Kinases assay. Following ...More data for this Ligand-Target Pair
Ligand Info
Affinity DataIC50: <1nMAssay Description:The enzymatic activity of compounds of the present invention was monitored measuring the formation of ADP using the ADP-GLO Kinases assay. Following ...More data for this Ligand-Target Pair
Ligand Info
Affinity DataIC50: <1nMAssay Description:The enzymatic activity of compounds of the present invention was monitored measuring the formation of ADP using the ADP-GLO Kinases assay. Following ...More data for this Ligand-Target Pair
Ligand Info
Affinity DataIC50: <1nMAssay Description:The enzymatic activity of compounds of the present invention was monitored measuring the formation of ADP using the ADP-GLO Kinases assay. Following ...More data for this Ligand-Target Pair
Ligand Info
Affinity DataIC50: <1nMAssay Description:The enzymatic activity of compounds of the present invention was monitored measuring the formation of ADP using the ADP-GLO Kinases assay. Following ...More data for this Ligand-Target Pair
Ligand Info

 3D Structure (crystal)
3D Structure (crystal)