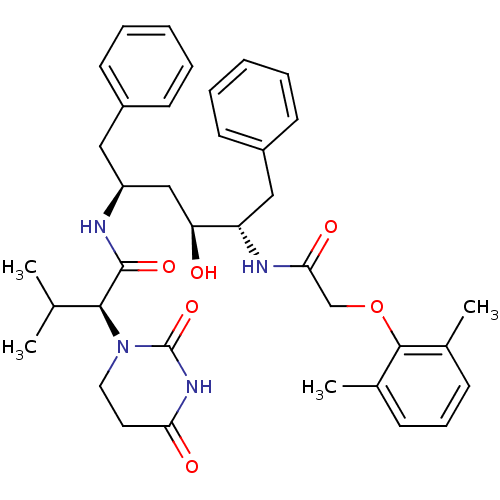
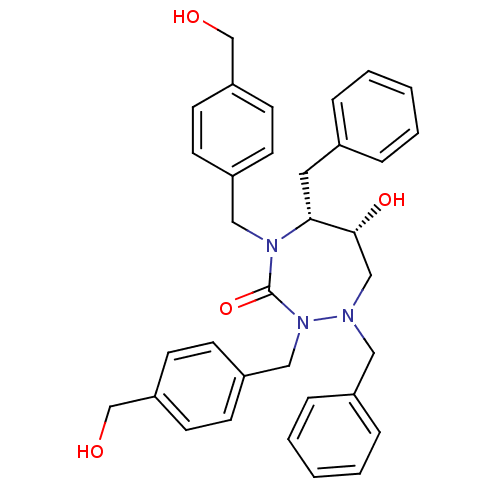
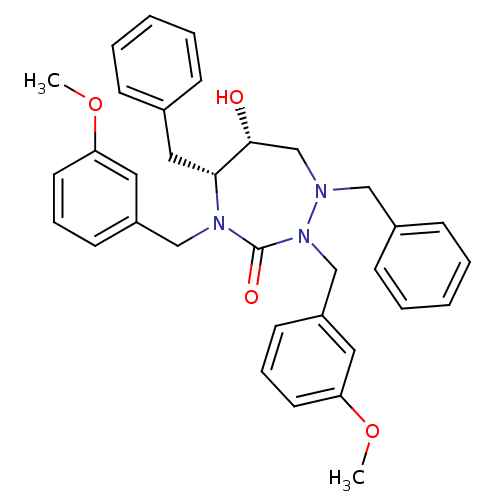
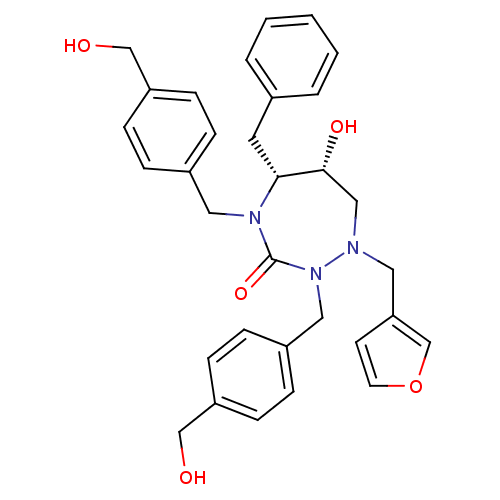
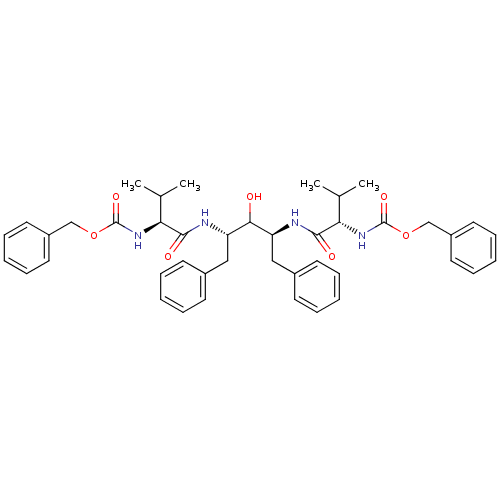
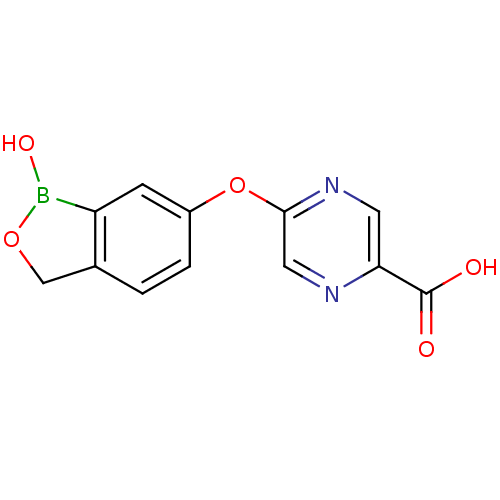
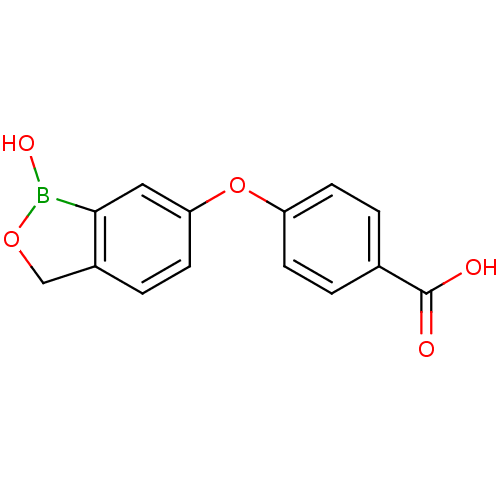
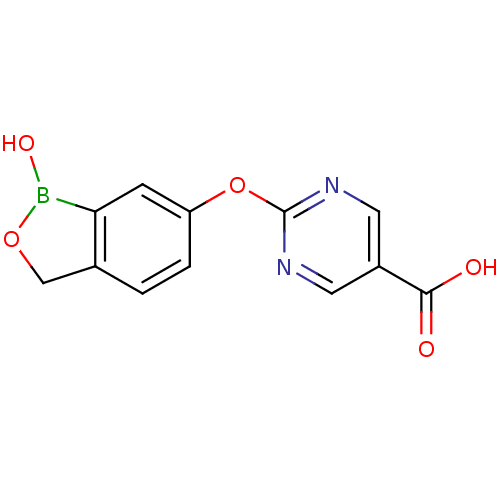
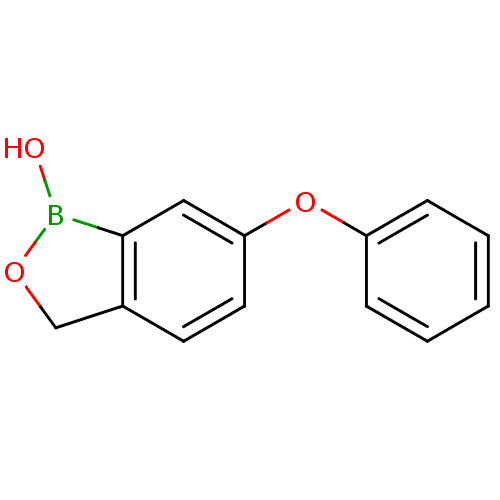
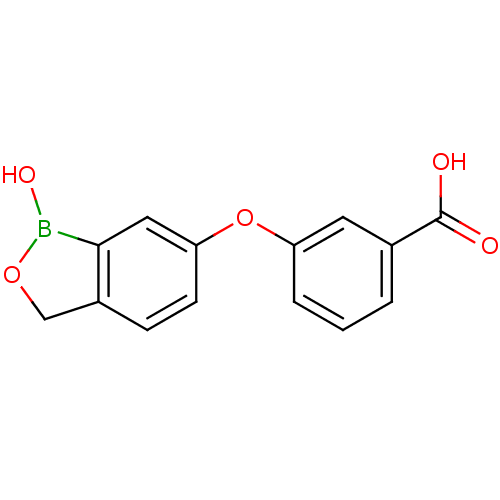
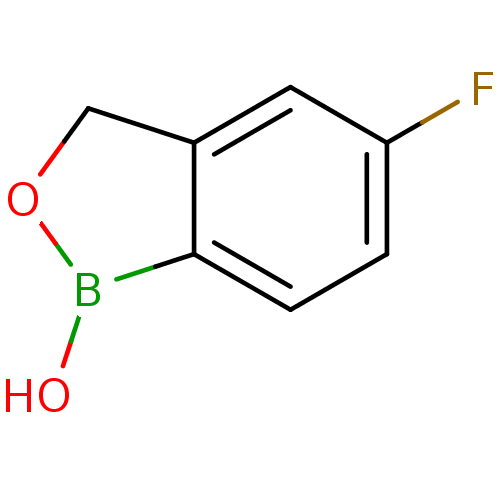
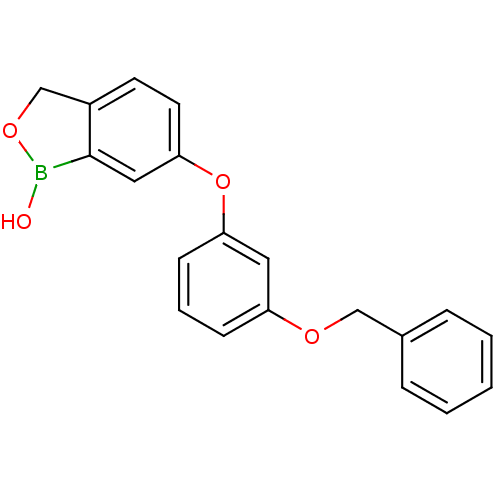
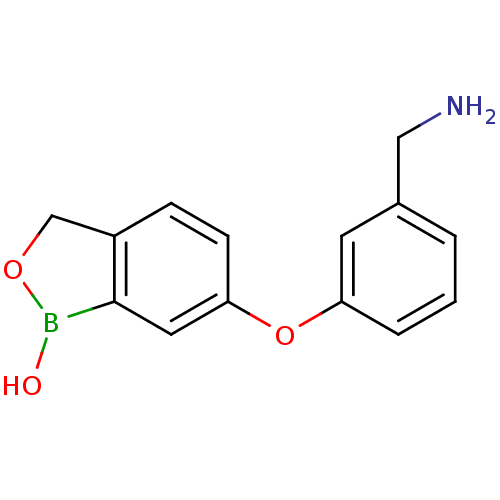
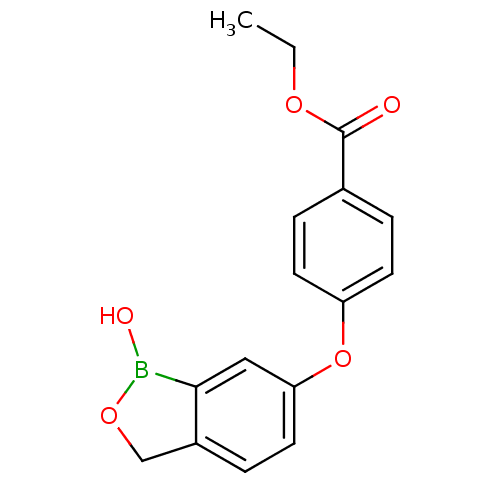
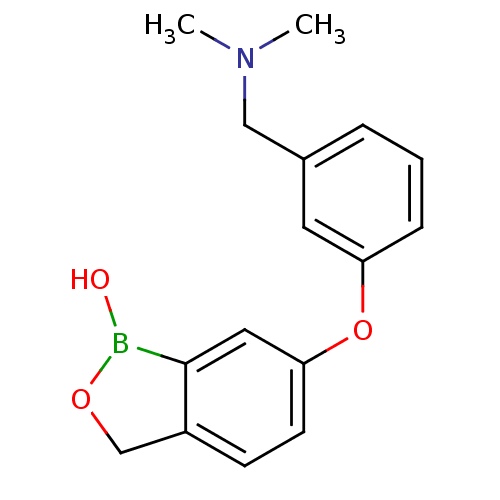
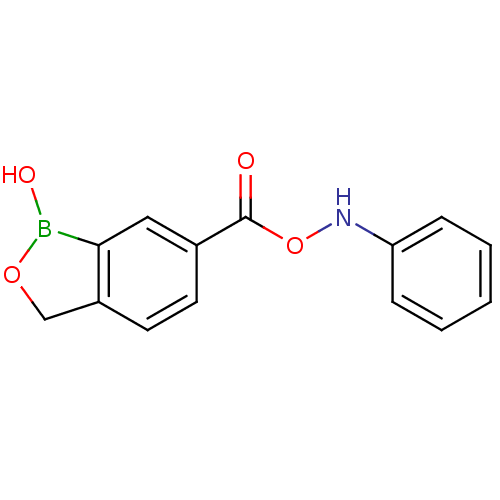
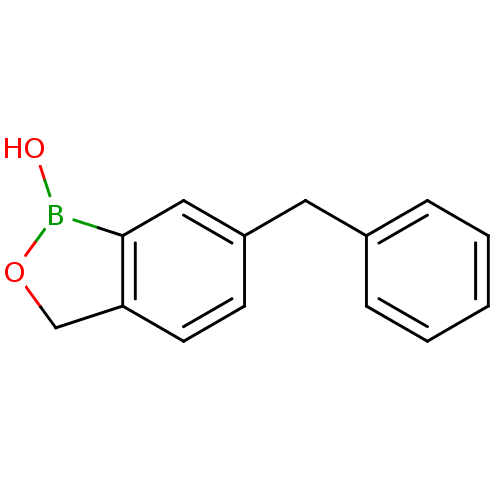
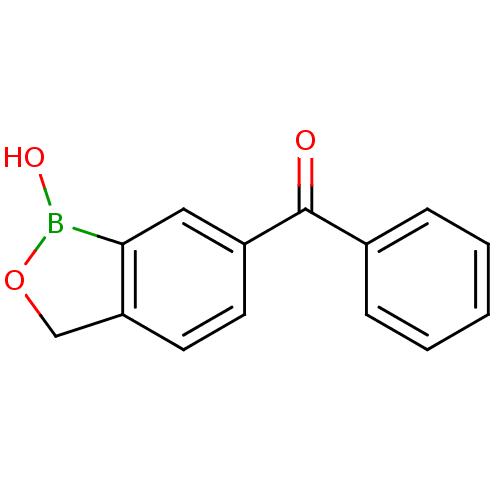
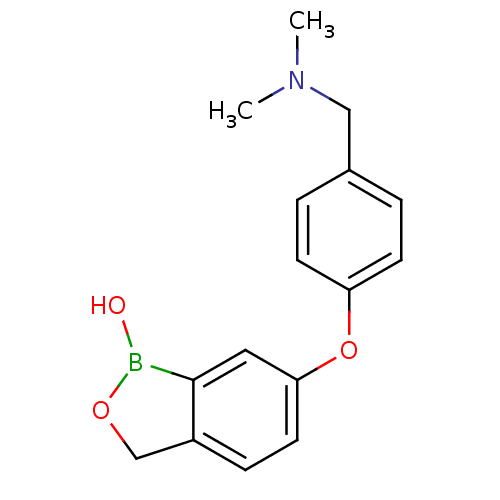
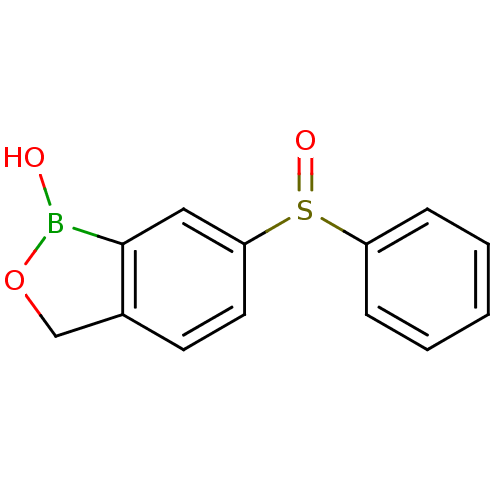
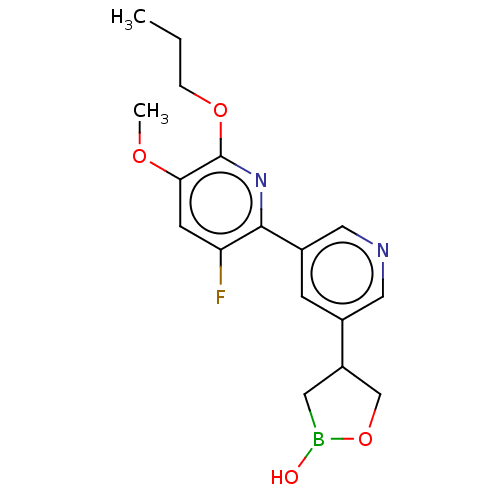
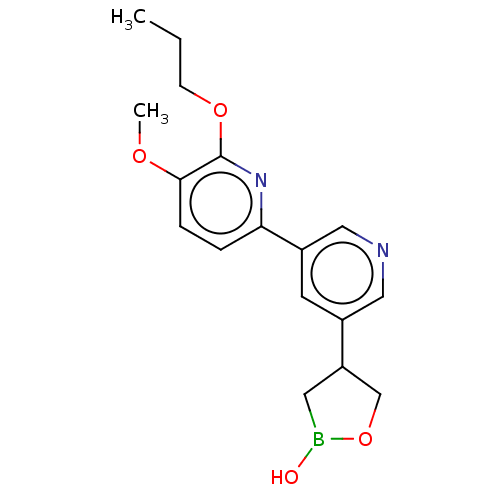
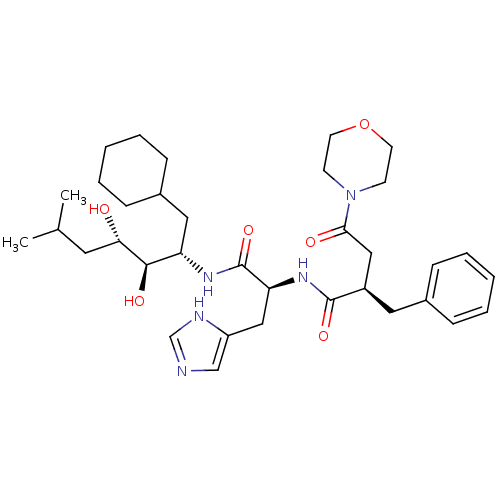
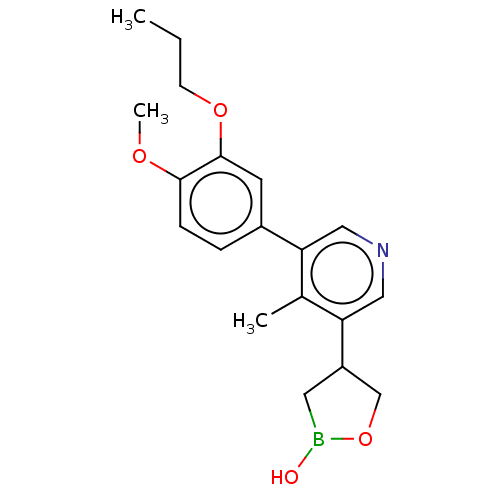
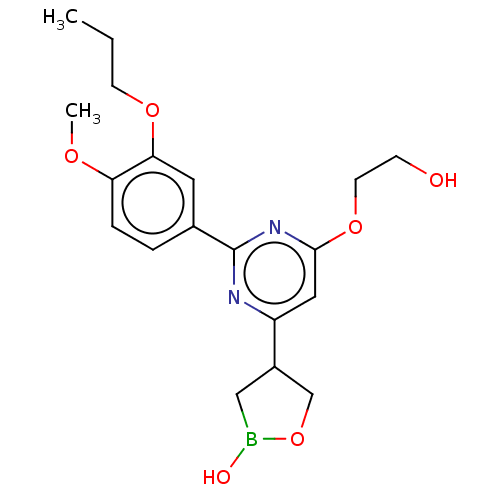
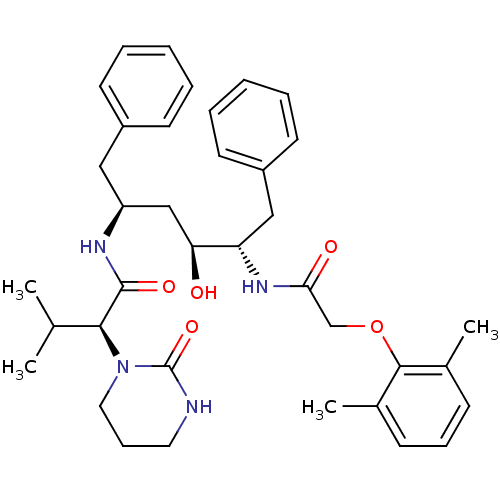
TargetGag-Pol polyprotein [489-587](Human immunodeficiency virus type 1)
Abbott Laboratories
Curated by ChEMBL
Abbott Laboratories
Curated by ChEMBL
Affinity DataKi: 0.00100nMAssay Description:Inhibitory activity against HIV proteaseMore data for this Ligand-Target Pair
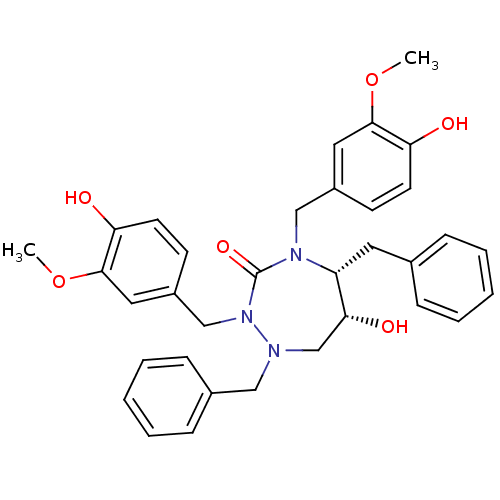
TargetGag-Pol polyprotein [489-587](Human immunodeficiency virus type 1)
Abbott Laboratories
Curated by ChEMBL
Abbott Laboratories
Curated by ChEMBL
Affinity DataKi: 0.00100nMAssay Description:Inhibitory activity against HIV proteaseMore data for this Ligand-Target Pair
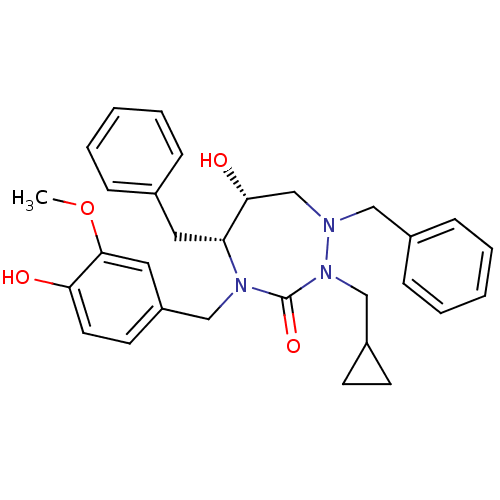
TargetGag-Pol polyprotein [489-587](Human immunodeficiency virus type 1)
Abbott Laboratories
Curated by ChEMBL
Abbott Laboratories
Curated by ChEMBL
Affinity DataKi: 0.00100nMAssay Description:Inhibitory activity against HIV proteaseMore data for this Ligand-Target Pair
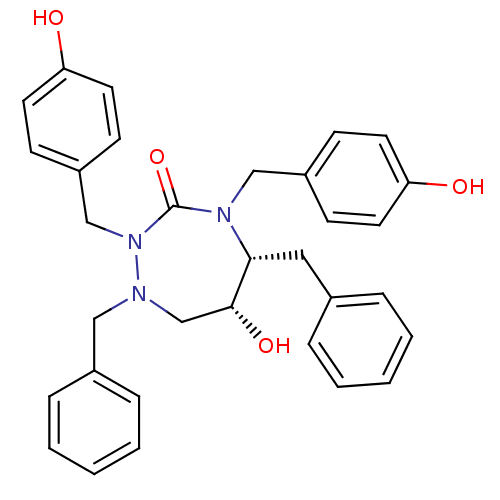
TargetDimer of Gag-Pol polyprotein [489-587](Human immunodeficiency virus type 1)
Abbott Laboratories
Abbott Laboratories
Affinity DataKi: 0.00500nM ΔG°: -65.6kJ/molepH: 4.7 T: 2°CAssay Description:HIV-1 protease activity was measured by a continuous fluorometric assay using the internally quenched fluorogenic substrate DABCYL-GABA-Ser-Gln-Tyr-P...More data for this Ligand-Target Pair
TargetDimer of Gag-Pol polyprotein [489-587](Human immunodeficiency virus type 1)
Abbott Laboratories
Abbott Laboratories
Affinity DataKi: 0.0620nM ΔG°: -59.2kJ/molepH: 4.7 T: 2°CAssay Description:HIV-1 protease activity was measured by a continuous fluorometric assay using the internally quenched fluorogenic substrate DABCYL-GABA-Ser-Gln-Tyr-P...More data for this Ligand-Target Pair
TargetDimer of Gag-Pol polyprotein [489-587](Human immunodeficiency virus type 1)
Abbott Laboratories
Abbott Laboratories
Affinity DataKi: 0.0700nM ΔG°: -58.9kJ/molepH: 4.7 T: 2°CAssay Description:HIV-1 protease activity was measured by a continuous fluorometric assay using the internally quenched fluorogenic substrate DABCYL-GABA-Ser-Gln-Tyr-P...More data for this Ligand-Target Pair
TargetDimer of Gag-Pol polyprotein [489-587](Human immunodeficiency virus type 1)
Abbott Laboratories
Abbott Laboratories
Affinity DataKi: 0.220nM ΔG°: -56.0kJ/molepH: 4.7 T: 2°CAssay Description:HIV-1 protease activity was measured by a continuous fluorometric assay using the internally quenched fluorogenic substrate DABCYL-GABA-Ser-Gln-Tyr-P...More data for this Ligand-Target Pair
TargetDimer of Gag-Pol polyprotein [489-587](Human immunodeficiency virus type 1)
Abbott Laboratories
Abbott Laboratories
Affinity DataKi: 0.220nM ΔG°: -56.0kJ/molepH: 4.7 T: 2°CAssay Description:HIV-1 protease activity was measured by a continuous fluorometric assay using the internally quenched fluorogenic substrate DABCYL-GABA-Ser-Gln-Tyr-P...More data for this Ligand-Target Pair
TargetDimer of Gag-Pol polyprotein [489-587](Human immunodeficiency virus type 1)
Abbott Laboratories
Abbott Laboratories
Affinity DataKi: 0.490nM ΔG°: -54.0kJ/molepH: 4.7 T: 2°CAssay Description:HIV-1 protease activity was measured by a continuous fluorometric assay using the internally quenched fluorogenic substrate DABCYL-GABA-Ser-Gln-Tyr-P...More data for this Ligand-Target Pair
TargetDimer of Gag-Pol polyprotein [489-587](Human immunodeficiency virus type 1)
Abbott Laboratories
Abbott Laboratories
Affinity DataKi: 2nM ΔG°: -50.5kJ/molepH: 4.7 T: 2°CAssay Description:HIV-1 protease activity was measured by a continuous fluorometric assay using the internally quenched fluorogenic substrate DABCYL-GABA-Ser-Gln-Tyr-P...More data for this Ligand-Target Pair
TargetDimer of Gag-Pol polyprotein [489-587](Human immunodeficiency virus type 1)
Abbott Laboratories
Abbott Laboratories
Affinity DataKi: 4.5nM ΔG°: -48.4kJ/molepH: 4.7 T: 2°CAssay Description:HIV-1 protease activity was measured by a continuous fluorometric assay using the internally quenched fluorogenic substrate DABCYL-GABA-Ser-Gln-Tyr-P...More data for this Ligand-Target Pair
Affinity DataKi: 20nMAssay Description:Inhibition of Enterobacter cloacae P99 beta-lactamase AmpC P99 using nitrocefin as substrate after 30 mins by spectrophotometric analysisMore data for this Ligand-Target Pair
Affinity DataKi: 20nMAssay Description:Inhibition of Enterobacter cloacae P99 beta-lactamase AmpC P99 using nitrocefin as substrate after 30 mins by spectrophotometric analysisMore data for this Ligand-Target Pair
Affinity DataKi: 80nMAssay Description:Inhibition of Enterobacter cloacae P99 beta-lactamase AmpC P99 using nitrocefin as substrate after 30 mins by spectrophotometric analysisMore data for this Ligand-Target Pair
Affinity DataKi: 710nMAssay Description:Inhibition of Enterobacter cloacae P99 beta-lactamase AmpC P99 using nitrocefin as substrate after 30 mins by spectrophotometric analysisMore data for this Ligand-Target Pair
Affinity DataKi: 1.35E+3nMAssay Description:Inhibition of Enterobacter cloacae P99 beta-lactamase AmpC P99 using nitrocefin as substrate after 30 mins by spectrophotometric analysisMore data for this Ligand-Target Pair
Affinity DataKi: 1.44E+3nMAssay Description:Inhibition of Enterobacter cloacae P99 beta-lactamase AmpC P99 using nitrocefin as substrate after 30 mins by spectrophotometric analysisMore data for this Ligand-Target Pair
TargetLeucine--tRNA ligase, cytoplasmic(Saccharomyces cerevisiae S288c)
Anacor Pharmaceuticals
Curated by ChEMBL
Anacor Pharmaceuticals
Curated by ChEMBL
Affinity DataKi: 1.85E+3nMAssay Description:Inhibition of Saccharomyces cerevisiae cytoplasmic leucyl-tRNA synthetase after 20 minsMore data for this Ligand-Target Pair
Affinity DataKi: 2.27E+3nMAssay Description:Inhibition of Enterobacter cloacae P99 beta-lactamase AmpC P99 using nitrocefin as substrate after 30 mins by spectrophotometric analysisMore data for this Ligand-Target Pair
Affinity DataKi: 2.75E+3nMAssay Description:Inhibition of Enterobacter cloacae P99 beta-lactamase AmpC P99 using nitrocefin as substrate after 30 mins by spectrophotometric analysisMore data for this Ligand-Target Pair
Affinity DataKi: 3.33E+3nMAssay Description:Inhibition of Enterobacter cloacae P99 beta-lactamase AmpC P99 using nitrocefin as substrate after 30 mins by spectrophotometric analysisMore data for this Ligand-Target Pair
Affinity DataKi: 4.73E+3nMAssay Description:Inhibition of Enterobacter cloacae P99 beta-lactamase AmpC P99 using nitrocefin as substrate after 30 mins by spectrophotometric analysisMore data for this Ligand-Target Pair
Affinity DataKi: 5.55E+3nMAssay Description:Inhibition of Enterobacter cloacae P99 beta-lactamase AmpC P99 using nitrocefin as substrate after 30 mins by spectrophotometric analysisMore data for this Ligand-Target Pair
Affinity DataKi: 6.00E+3nMAssay Description:Inhibition of Enterobacter cloacae P99 beta-lactamase AmpC P99 using nitrocefin as substrate after 30 mins by spectrophotometric analysisMore data for this Ligand-Target Pair
Affinity DataKi: 7.28E+3nMAssay Description:Inhibition of Enterobacter cloacae P99 beta-lactamase AmpC P99 using nitrocefin as substrate after 30 mins by spectrophotometric analysisMore data for this Ligand-Target Pair
Affinity DataKi: 7.72E+3nMAssay Description:Inhibition of Enterobacter cloacae P99 beta-lactamase AmpC P99 using nitrocefin as substrate after 30 mins by spectrophotometric analysisMore data for this Ligand-Target Pair
Affinity DataKi: 8.40E+3nMAssay Description:Inhibition of Enterobacter cloacae P99 beta-lactamase AmpC P99 using nitrocefin as substrate after 30 mins by spectrophotometric analysisMore data for this Ligand-Target Pair
Affinity DataKi: 8.73E+3nMAssay Description:Inhibition of Enterobacter cloacae P99 beta-lactamase AmpC P99 using nitrocefin as substrate after 30 mins by spectrophotometric analysisMore data for this Ligand-Target Pair
Affinity DataKi: 9.09E+3nMAssay Description:Inhibition of Enterobacter cloacae P99 beta-lactamase AmpC P99 using nitrocefin as substrate after 30 mins by spectrophotometric analysisMore data for this Ligand-Target Pair
Affinity DataKi: 1.33E+4nMAssay Description:Inhibition of Enterobacter cloacae P99 beta-lactamase AmpC P99 using nitrocefin as substrate after 30 mins by spectrophotometric analysisMore data for this Ligand-Target Pair
Affinity DataKi: 1.60E+4nMAssay Description:Inhibition of Enterobacter cloacae P99 beta-lactamase AmpC P99 using nitrocefin as substrate after 30 mins by spectrophotometric analysisMore data for this Ligand-Target Pair
Affinity DataKi: 1.98E+4nMAssay Description:Inhibition of Enterobacter cloacae P99 beta-lactamase AmpC P99 using nitrocefin as substrate after 30 mins by spectrophotometric analysisMore data for this Ligand-Target Pair
Affinity DataKi: 2.85E+4nMAssay Description:Inhibition of Enterobacter cloacae P99 beta-lactamase AmpC P99 using nitrocefin as substrate after 30 mins by spectrophotometric analysisMore data for this Ligand-Target Pair
TargetLeucine--tRNA ligase, cytoplasmic(Saccharomyces cerevisiae S288c)
Anacor Pharmaceuticals
Curated by ChEMBL
Anacor Pharmaceuticals
Curated by ChEMBL
Affinity DataKi: 3.14E+4nMAssay Description:Inhibition of Saccharomyces cerevisiae cytoplasmic leucyl-tRNA synthetase after 2 minsMore data for this Ligand-Target Pair
Affinity DataKi: 4.06E+4nMAssay Description:Inhibition of Enterobacter cloacae P99 beta-lactamase AmpC P99 using nitrocefin as substrate after 30 mins by spectrophotometric analysisMore data for this Ligand-Target Pair
Affinity DataKi: 4.66E+4nMAssay Description:Inhibition of Enterobacter cloacae P99 beta-lactamase AmpC P99 using nitrocefin as substrate after 30 mins by spectrophotometric analysisMore data for this Ligand-Target Pair
Affinity DataKi: 5.80E+4nMAssay Description:Inhibition of Enterobacter cloacae P99 beta-lactamase AmpC P99 using nitrocefin as substrate after 30 mins by spectrophotometric analysisMore data for this Ligand-Target Pair
Affinity DataKi: 6.32E+4nMAssay Description:Inhibition of Enterobacter cloacae P99 beta-lactamase AmpC P99 using nitrocefin as substrate after 30 mins by spectrophotometric analysisMore data for this Ligand-Target Pair
TargetIsoform PDE4B2 of cAMP-specific 3',5'-cyclic phosphodiesterase 4B(Homo sapiens (Human))
Pfizer
US Patent
Pfizer
US Patent
Affinity DataIC50: 0.0500nMAssay Description:Cytokine inhibitory activity is determined by measuring the effect of test agent on the release of the cytokines IL-4, IL-13 and IFNγ from human...More data for this Ligand-Target Pair
TargetcAMP-dependent protein kinase catalytic subunit alpha(Homo sapiens (Human))
Anacor Pharmaceuticals
US Patent
Anacor Pharmaceuticals
US Patent
Affinity DataIC50: 0.0550nMT: 2°CAssay Description:Phosphorylation of activity of ROCK1 and ROCK2 and those of other kinases, AKT1, GRK2, PKA, PKCa and RSK1 were performed by Reaction Biology (Malvern...More data for this Ligand-Target Pair
TargetIsoform PDE4B2 of cAMP-specific 3',5'-cyclic phosphodiesterase 4B(Homo sapiens (Human))
Pfizer
US Patent
Pfizer
US Patent
Affinity DataIC50: 0.120nMAssay Description:Cytokine inhibitory activity is determined by measuring the effect of test agent on the release of the cytokines IL-4, IL-13 and IFNγ from human...More data for this Ligand-Target Pair
Affinity DataIC50: 0.195nMT: 2°CAssay Description:Phosphorylation of activity of ROCK1 and ROCK2 and those of other kinases, AKT1, GRK2, PKA, PKCa and RSK1 were performed by Reaction Biology (Malvern...More data for this Ligand-Target Pair
TargetIsoform PDE4B2 of cAMP-specific 3',5'-cyclic phosphodiesterase 4B(Homo sapiens (Human))
Pfizer
US Patent
Pfizer
US Patent
Affinity DataIC50: 0.220nMAssay Description:Cytokine inhibitory activity is determined by measuring the effect of test agent on the release of the cytokines IL-4, IL-13 and IFNγ from human...More data for this Ligand-Target Pair
TargetIsoform PDE4B2 of cAMP-specific 3',5'-cyclic phosphodiesterase 4B(Homo sapiens (Human))
Pfizer
US Patent
Pfizer
US Patent
Affinity DataIC50: 0.240nMAssay Description:Cytokine inhibitory activity is determined by measuring the effect of test agent on the release of the cytokines IL-4, IL-13 and IFNγ from human...More data for this Ligand-Target Pair
TargetIsoform PDE4B2 of cAMP-specific 3',5'-cyclic phosphodiesterase 4B(Homo sapiens (Human))
Pfizer
US Patent
Pfizer
US Patent
Affinity DataIC50: 0.25nMAssay Description:Cytokine inhibitory activity is determined by measuring the effect of test agent on the release of the cytokines IL-4, IL-13 and IFNγ from human...More data for this Ligand-Target Pair
TargetIsoform PDE4B2 of cAMP-specific 3',5'-cyclic phosphodiesterase 4B(Homo sapiens (Human))
Pfizer
US Patent
Pfizer
US Patent
Affinity DataIC50: 0.280nMAssay Description:Cytokine inhibitory activity is determined by measuring the effect of test agent on the release of the cytokines IL-4, IL-13 and IFNγ from human...More data for this Ligand-Target Pair
TargetIsoform PDE4B2 of cAMP-specific 3',5'-cyclic phosphodiesterase 4B(Homo sapiens (Human))
Pfizer
US Patent
Pfizer
US Patent
Affinity DataIC50: 0.280nMAssay Description:Cytokine inhibitory activity is determined by measuring the effect of test agent on the release of the cytokines IL-4, IL-13 and IFNγ from human...More data for this Ligand-Target Pair
Affinity DataIC50: 0.290nMpH: 6.0Assay Description:Inhibition of purified human renin (pH 6.0)More data for this Ligand-Target Pair
TargetIsoform PDE4B2 of cAMP-specific 3',5'-cyclic phosphodiesterase 4B(Homo sapiens (Human))
Pfizer
US Patent
Pfizer
US Patent
Affinity DataIC50: 0.290nMAssay Description:Cytokine inhibitory activity is determined by measuring the effect of test agent on the release of the cytokines IL-4, IL-13 and IFNγ from human...More data for this Ligand-Target Pair


 3D Structure (crystal)
3D Structure (crystal)