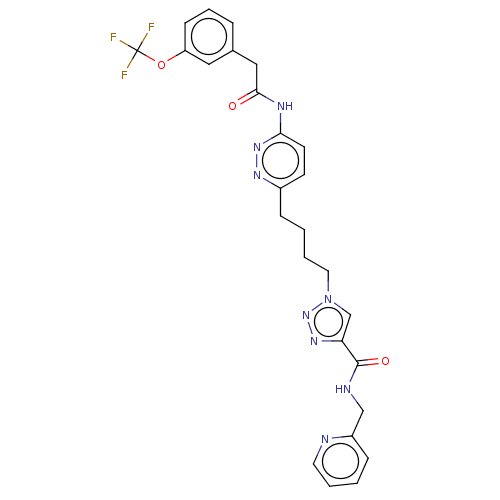
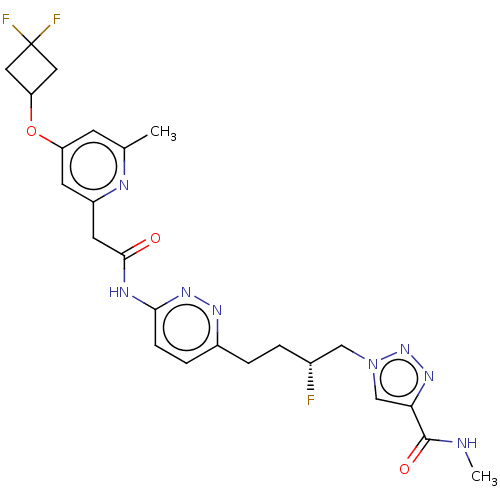
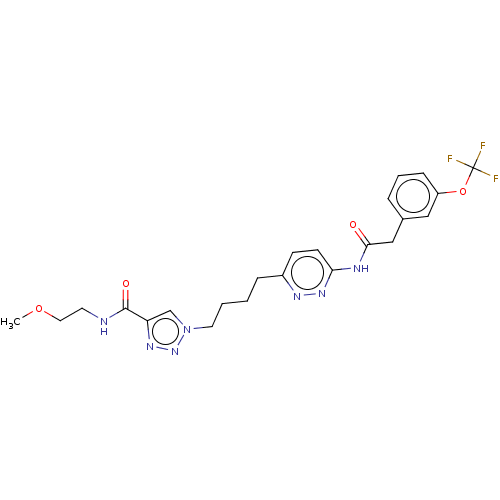
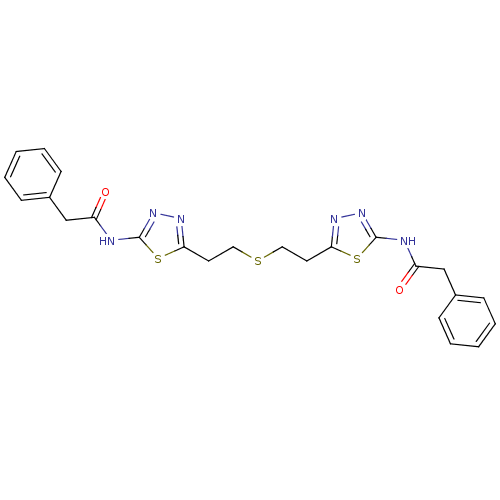
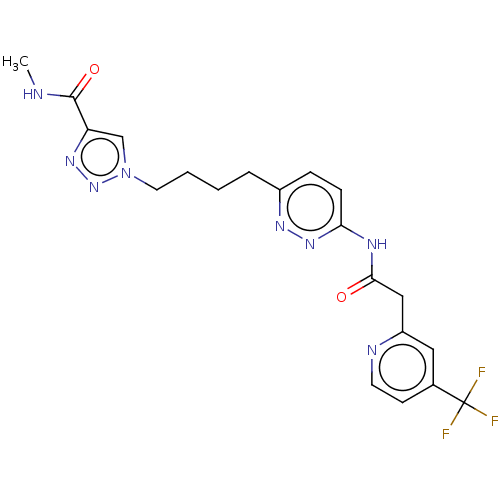
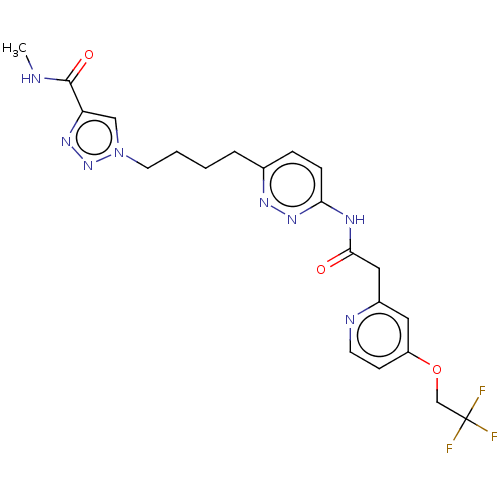
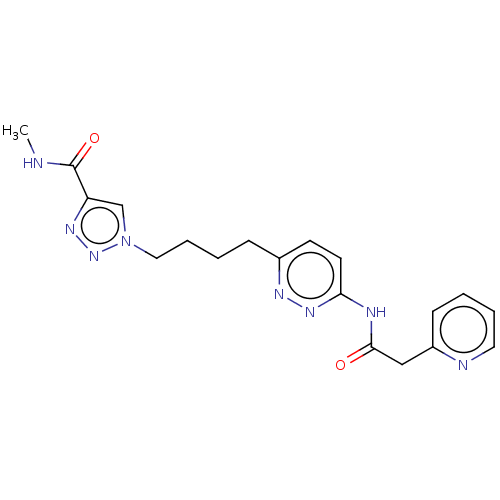
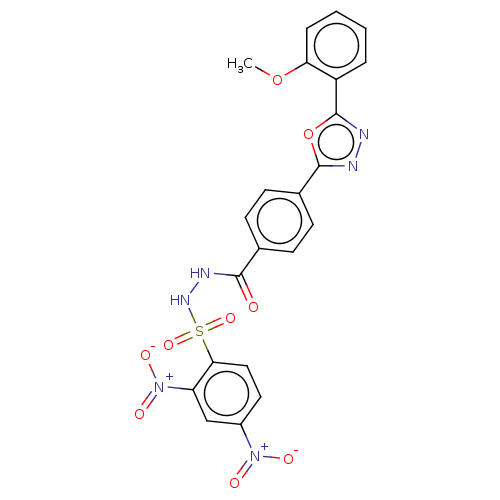
TargetSomatostatin receptor type 2(Homo sapiens (Human))
Novartis Pharma Research
Curated by PDSP Ki Database
Novartis Pharma Research
Curated by PDSP Ki Database
TargetSomatostatin receptor type 3(Homo sapiens (Human))
Novartis Pharma Research
Curated by PDSP Ki Database
Novartis Pharma Research
Curated by PDSP Ki Database
TargetSomatostatin receptor type 2(Homo sapiens (Human))
Novartis Pharma Research
Curated by PDSP Ki Database
Novartis Pharma Research
Curated by PDSP Ki Database
TargetSomatostatin receptor type 5(Homo sapiens (Human))
Novartis Pharma Research
Curated by PDSP Ki Database
Novartis Pharma Research
Curated by PDSP Ki Database
TargetSomatostatin receptor type 2(Homo sapiens (Human))
Novartis Pharma Research
Curated by PDSP Ki Database
Novartis Pharma Research
Curated by PDSP Ki Database
TargetSomatostatin receptor type 1(Homo sapiens (Human))
Novartis Pharma Research
Curated by PDSP Ki Database
Novartis Pharma Research
Curated by PDSP Ki Database
TargetSomatostatin receptor type 4(Homo sapiens (Human))
Novartis Pharma Research
Curated by PDSP Ki Database
Novartis Pharma Research
Curated by PDSP Ki Database
TargetSomatostatin receptor type 5(Homo sapiens (Human))
Novartis Pharma Research
Curated by PDSP Ki Database
Novartis Pharma Research
Curated by PDSP Ki Database
TargetSomatostatin receptor type 5(Homo sapiens (Human))
Novartis Pharma Research
Curated by PDSP Ki Database
Novartis Pharma Research
Curated by PDSP Ki Database
TargetSomatostatin receptor type 3(Homo sapiens (Human))
Novartis Pharma Research
Curated by PDSP Ki Database
Novartis Pharma Research
Curated by PDSP Ki Database
TargetSomatostatin receptor type 3(Homo sapiens (Human))
Novartis Pharma Research
Curated by PDSP Ki Database
Novartis Pharma Research
Curated by PDSP Ki Database
TargetSomatostatin receptor type 1(Homo sapiens (Human))
Novartis Pharma Research
Curated by PDSP Ki Database
Novartis Pharma Research
Curated by PDSP Ki Database
TargetSomatostatin receptor type 4(Homo sapiens (Human))
Novartis Pharma Research
Curated by PDSP Ki Database
Novartis Pharma Research
Curated by PDSP Ki Database
TargetSomatostatin receptor type 4(Homo sapiens (Human))
Novartis Pharma Research
Curated by PDSP Ki Database
Novartis Pharma Research
Curated by PDSP Ki Database
TargetCarnitine O-palmitoyltransferase 1, liver isoform(Rattus norvegicus)
Sandoz Research Institute
Curated by ChEMBL
Sandoz Research Institute
Curated by ChEMBL
Affinity DataKi: 3.60E+3nMAssay Description:In vitro inhibition of carnitine palmitoyltransferase I withrespect to palmitoyl CoA in ratMore data for this Ligand-Target Pair
TargetGlutaminase kidney isoform, mitochondrial(Homo sapiens (Human))
The University Of Texas Md Anderson Cancer Center
Curated by ChEMBL
The University Of Texas Md Anderson Cancer Center
Curated by ChEMBL
Affinity DataIC50: 6nMAssay Description:Inhibition of recombinant human GLS1 using glutamine as substrate preincubated for 10 mins followed by substrate addition and measured after 20 mins ...More data for this Ligand-Target Pair
TargetGlutaminase kidney isoform, mitochondrial(Homo sapiens (Human))
The University Of Texas Md Anderson Cancer Center
Curated by ChEMBL
The University Of Texas Md Anderson Cancer Center
Curated by ChEMBL
Affinity DataIC50: 6nMAssay Description:Inhibition of recombinant human GLS1 using glutamine as substrate preincubated for 10 mins followed by substrate addition and measured after 20 mins ...More data for this Ligand-Target Pair
TargetGlutaminase kidney isoform, mitochondrial(Homo sapiens (Human))
The University Of Texas Md Anderson Cancer Center
Curated by ChEMBL
The University Of Texas Md Anderson Cancer Center
Curated by ChEMBL
Affinity DataIC50: 10nMAssay Description:Inhibition of recombinant human GLS1 using glutamine as substrate preincubated for 10 mins followed by substrate addition and measured after 20 mins ...More data for this Ligand-Target Pair
TargetGlutaminase kidney isoform, mitochondrial(Homo sapiens (Human))
The University Of Texas Md Anderson Cancer Center
Curated by ChEMBL
The University Of Texas Md Anderson Cancer Center
Curated by ChEMBL
Affinity DataIC50: 10nMAssay Description:Inhibition of recombinant human GLS1 using glutamine as substrate preincubated for 10 mins followed by substrate addition and measured after 20 mins ...More data for this Ligand-Target Pair
TargetGlutaminase kidney isoform, mitochondrial(Homo sapiens (Human))
The University Of Texas Md Anderson Cancer Center
Curated by ChEMBL
The University Of Texas Md Anderson Cancer Center
Curated by ChEMBL
Affinity DataIC50: 14nMAssay Description:Inhibition of recombinant human GLS1 using glutamine as substrate preincubated for 10 mins followed by substrate addition and measured after 20 mins ...More data for this Ligand-Target Pair
TargetGlutaminase kidney isoform, mitochondrial(Homo sapiens (Human))
The University Of Texas Md Anderson Cancer Center
Curated by ChEMBL
The University Of Texas Md Anderson Cancer Center
Curated by ChEMBL
Affinity DataIC50: 14nMAssay Description:Inhibition of recombinant human GLS1 using glutamine as substrate preincubated for 10 mins followed by substrate addition and measured after 20 mins ...More data for this Ligand-Target Pair
TargetGlutaminase kidney isoform, mitochondrial(Homo sapiens (Human))
The University Of Texas Md Anderson Cancer Center
Curated by ChEMBL
The University Of Texas Md Anderson Cancer Center
Curated by ChEMBL
Affinity DataIC50: 14nMAssay Description:Inhibition of recombinant human GLS1 using glutamine as substrate preincubated for 10 mins followed by substrate addition and measured after 20 mins ...More data for this Ligand-Target Pair
TargetGlutaminase kidney isoform, mitochondrial(Homo sapiens (Human))
The University Of Texas Md Anderson Cancer Center
Curated by ChEMBL
The University Of Texas Md Anderson Cancer Center
Curated by ChEMBL
Affinity DataIC50: 16nMAssay Description:Inhibition of GLS1 in human A549 cells assessed as reduction in conversion of glutamine to glutamate measured after 24 hrsMore data for this Ligand-Target Pair
TargetGlutaminase kidney isoform, mitochondrial(Homo sapiens (Human))
The University Of Texas Md Anderson Cancer Center
Curated by ChEMBL
The University Of Texas Md Anderson Cancer Center
Curated by ChEMBL
Affinity DataIC50: 21nMAssay Description:Inhibition of recombinant human GLS1 using glutamine as substrate preincubated for 10 mins followed by substrate addition and measured after 20 mins ...More data for this Ligand-Target Pair
TargetGlutaminase kidney isoform, mitochondrial(Homo sapiens (Human))
The University Of Texas Md Anderson Cancer Center
Curated by ChEMBL
The University Of Texas Md Anderson Cancer Center
Curated by ChEMBL
Affinity DataIC50: 22nMAssay Description:Inhibition of recombinant human GLS1 using glutamine as substrate preincubated for 10 mins followed by substrate addition and measured after 20 mins ...More data for this Ligand-Target Pair
TargetGlutaminase kidney isoform, mitochondrial(Homo sapiens (Human))
The University Of Texas Md Anderson Cancer Center
Curated by ChEMBL
The University Of Texas Md Anderson Cancer Center
Curated by ChEMBL
Affinity DataIC50: 23nMAssay Description:Inhibition of recombinant human GLS1 using glutamine as substrate preincubated for 10 mins followed by substrate addition and measured after 20 mins ...More data for this Ligand-Target Pair
TargetGlutaminase kidney isoform, mitochondrial(Homo sapiens (Human))
The University Of Texas Md Anderson Cancer Center
Curated by ChEMBL
The University Of Texas Md Anderson Cancer Center
Curated by ChEMBL
Affinity DataIC50: 25nMAssay Description:Inhibition of recombinant human GLS1 using glutamine as substrate preincubated for 10 mins followed by substrate addition and measured after 20 mins ...More data for this Ligand-Target Pair
TargetGlutaminase kidney isoform, mitochondrial(Homo sapiens (Human))
The University Of Texas Md Anderson Cancer Center
Curated by ChEMBL
The University Of Texas Md Anderson Cancer Center
Curated by ChEMBL
Affinity DataIC50: 25nMAssay Description:Inhibition of recombinant human GLS1 using glutamine as substrate preincubated for 10 mins followed by substrate addition and measured after 20 mins ...More data for this Ligand-Target Pair
TargetGlutaminase kidney isoform, mitochondrial(Homo sapiens (Human))
The University Of Texas Md Anderson Cancer Center
Curated by ChEMBL
The University Of Texas Md Anderson Cancer Center
Curated by ChEMBL
Affinity DataIC50: 31nMAssay Description:Inhibition of recombinant human GLS1 using glutamine as substrate preincubated for 10 mins followed by substrate addition and measured after 20 mins ...More data for this Ligand-Target Pair
TargetGlutaminase kidney isoform, mitochondrial(Homo sapiens (Human))
The University Of Texas Md Anderson Cancer Center
Curated by ChEMBL
The University Of Texas Md Anderson Cancer Center
Curated by ChEMBL
Affinity DataIC50: 32nMAssay Description:Inhibition of recombinant human GLS1 using glutamine as substrate preincubated for 10 mins followed by substrate addition and measured after 20 mins ...More data for this Ligand-Target Pair
TargetGlutaminase kidney isoform, mitochondrial(Homo sapiens (Human))
The University Of Texas Md Anderson Cancer Center
Curated by ChEMBL
The University Of Texas Md Anderson Cancer Center
Curated by ChEMBL
Affinity DataIC50: 32nMAssay Description:Inhibition of recombinant human GLS1 using glutamine as substrate preincubated for 10 mins followed by substrate addition and measured after 20 mins ...More data for this Ligand-Target Pair
TargetGlutaminase kidney isoform, mitochondrial(Homo sapiens (Human))
The University Of Texas Md Anderson Cancer Center
Curated by ChEMBL
The University Of Texas Md Anderson Cancer Center
Curated by ChEMBL
Affinity DataIC50: 42nMAssay Description:Inhibition of recombinant human GLS1 using glutamine as substrate preincubated for 10 mins followed by substrate addition and measured after 20 mins ...More data for this Ligand-Target Pair
TargetGlutaminase kidney isoform, mitochondrial(Homo sapiens (Human))
The University Of Texas Md Anderson Cancer Center
Curated by ChEMBL
The University Of Texas Md Anderson Cancer Center
Curated by ChEMBL
Affinity DataIC50: 44nMAssay Description:Inhibition of recombinant human GLS1 using glutamine as substrate preincubated for 10 mins followed by substrate addition and measured after 20 mins ...More data for this Ligand-Target Pair
TargetGlutaminase kidney isoform, mitochondrial(Homo sapiens (Human))
The University Of Texas Md Anderson Cancer Center
Curated by ChEMBL
The University Of Texas Md Anderson Cancer Center
Curated by ChEMBL
Affinity DataIC50: 46nMAssay Description:Inhibition of recombinant human GLS1 using glutamine as substrate preincubated for 10 mins followed by substrate addition and measured after 20 mins ...More data for this Ligand-Target Pair
TargetGlutaminase kidney isoform, mitochondrial(Homo sapiens (Human))
The University Of Texas Md Anderson Cancer Center
Curated by ChEMBL
The University Of Texas Md Anderson Cancer Center
Curated by ChEMBL
Affinity DataIC50: 49nMAssay Description:Inhibition of recombinant human GLS1 using glutamine as substrate preincubated for 10 mins followed by substrate addition and measured after 20 mins ...More data for this Ligand-Target Pair
TargetGlutaminase kidney isoform, mitochondrial(Homo sapiens (Human))
The University Of Texas Md Anderson Cancer Center
Curated by ChEMBL
The University Of Texas Md Anderson Cancer Center
Curated by ChEMBL
Affinity DataIC50: 62nMAssay Description:Inhibition of recombinant human GLS1 using glutamine as substrate preincubated for 10 mins followed by substrate addition and measured after 20 mins ...More data for this Ligand-Target Pair
TargetGlutaminase kidney isoform, mitochondrial(Homo sapiens (Human))
The University Of Texas Md Anderson Cancer Center
Curated by ChEMBL
The University Of Texas Md Anderson Cancer Center
Curated by ChEMBL
Affinity DataIC50: 67nMAssay Description:Inhibition of recombinant human GLS1 using glutamine as substrate preincubated for 10 mins followed by substrate addition and measured after 20 mins ...More data for this Ligand-Target Pair
TargetGlutaminase kidney isoform, mitochondrial(Homo sapiens (Human))
The University Of Texas Md Anderson Cancer Center
Curated by ChEMBL
The University Of Texas Md Anderson Cancer Center
Curated by ChEMBL
Affinity DataIC50: 71nMAssay Description:Inhibition of recombinant human GLS1 using glutamine as substrate preincubated for 10 mins followed by substrate addition and measured after 20 mins ...More data for this Ligand-Target Pair
TargetGlutaminase kidney isoform, mitochondrial(Homo sapiens (Human))
The University Of Texas Md Anderson Cancer Center
Curated by ChEMBL
The University Of Texas Md Anderson Cancer Center
Curated by ChEMBL
Affinity DataIC50: 88nMAssay Description:Inhibition of recombinant human GLS1 using glutamine as substrate preincubated for 10 mins followed by substrate addition and measured after 20 mins ...More data for this Ligand-Target Pair
TargetGlutaminase kidney isoform, mitochondrial(Homo sapiens (Human))
The University Of Texas Md Anderson Cancer Center
Curated by ChEMBL
The University Of Texas Md Anderson Cancer Center
Curated by ChEMBL
Affinity DataIC50: 100nMAssay Description:Inhibition of recombinant human GLS1 using glutamine as substrate preincubated for 10 mins followed by substrate addition and measured after 20 mins ...More data for this Ligand-Target Pair
TargetGlutaminase kidney isoform, mitochondrial(Homo sapiens (Human))
The University Of Texas Md Anderson Cancer Center
Curated by ChEMBL
The University Of Texas Md Anderson Cancer Center
Curated by ChEMBL
Affinity DataIC50: 118nMAssay Description:Inhibition of recombinant human GLS1 using glutamine as substrate preincubated for 10 mins followed by substrate addition and measured after 20 mins ...More data for this Ligand-Target Pair
Affinity DataIC50: 500nMAssay Description:β-glucuronidase activity was determined in accordance to method used by Taha et al. [Taha et al., Bioorg. Med. Chem., 23:7394-7404] by measuring...More data for this Ligand-Target Pair
Affinity DataIC50: 2.40E+3nMAssay Description:β-glucuronidase activity was determined in accordance to method used by Taha et al. [Taha et al., Bioorg. Med. Chem., 23:7394-7404] by measuring...More data for this Ligand-Target Pair
Affinity DataIC50: 2.40E+3nMAssay Description:β-glucuronidase activity was determined by measuring absorbance at 405 nm of p-nitrophenol formed substrate by spectrophotometric method. 250 &#...More data for this Ligand-Target Pair
Affinity DataIC50: 2.50E+3nMAssay Description:β-glucuronidase activity was determined in accordance to method used by Taha et al. [Taha et al., Bioorg. Med. Chem., 23:7394-7404] by measuring...More data for this Ligand-Target Pair
Affinity DataIC50: 2.70E+3nMAssay Description:β-glucuronidase activity was determined in accordance to method used by Taha et al. [Taha et al., Bioorg. Med. Chem., 23:7394-7404] by measuring...More data for this Ligand-Target Pair
Affinity DataIC50: 6.34E+3nMAssay Description:β-glucuronidase activity was determined by measuring absorbance at 405 nm of p-nitrophenol formed substrate by spectrophotometric method. 250 &#...More data for this Ligand-Target Pair
Affinity DataIC50: 6.50E+3nMAssay Description:β-glucuronidase activity was determined in accordance to method used by Taha et al. [Taha et al., Bioorg. Med. Chem., 23:7394-7404] by measuring...More data for this Ligand-Target Pair
Affinity DataIC50: 7.50E+3nMAssay Description:β-glucuronidase activity was determined in accordance to method used by Taha et al. [Taha et al., Bioorg. Med. Chem., 23:7394-7404] by measuring...More data for this Ligand-Target Pair
Affinity DataIC50: 8.70E+3nMAssay Description:β-glucuronidase activity was determined in accordance to method used by Taha et al. [Taha et al., Bioorg. Med. Chem., 23:7394-7404] by measuring...More data for this Ligand-Target Pair

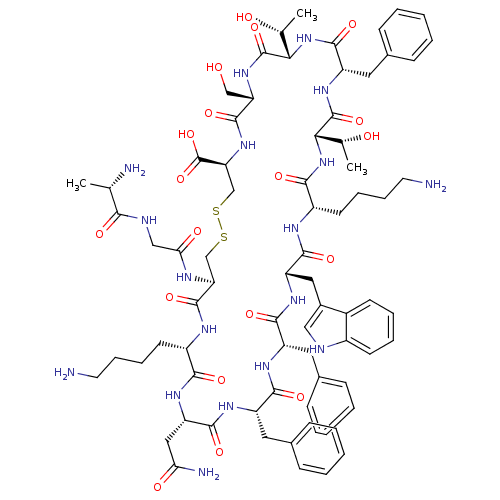
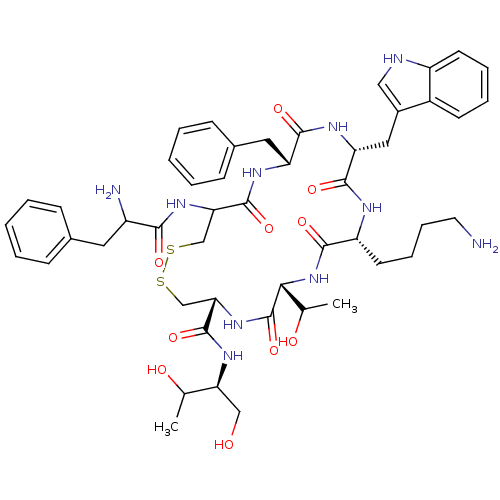
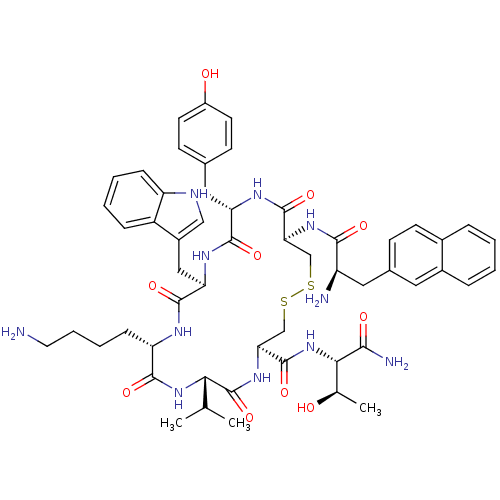
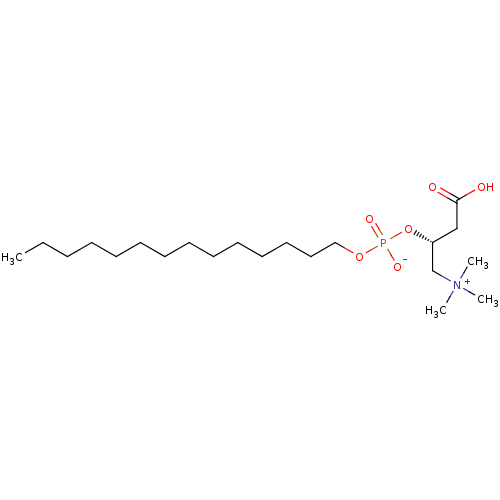

 3D Structure (crystal)
3D Structure (crystal)