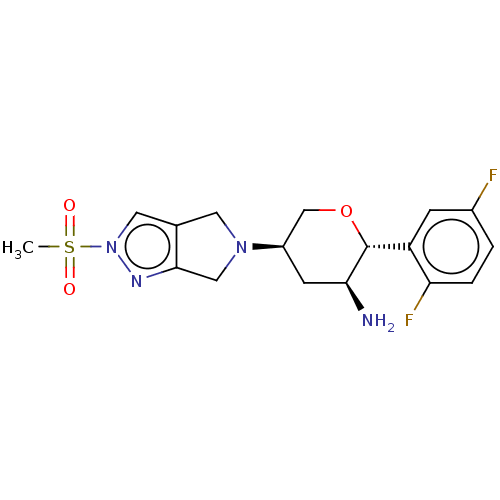
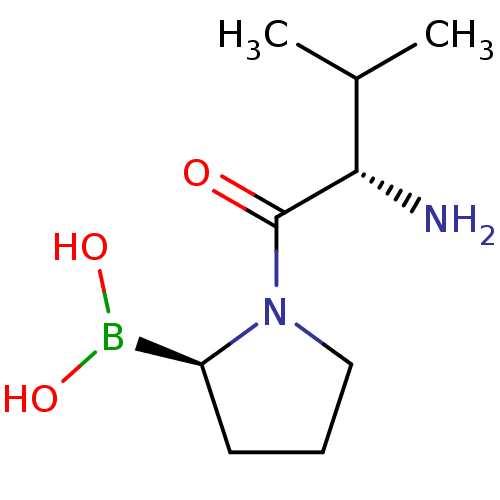
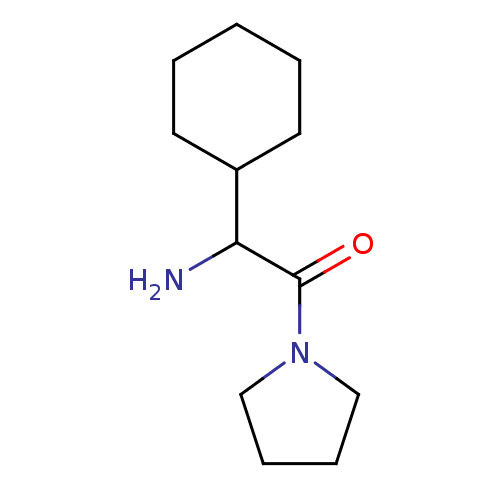
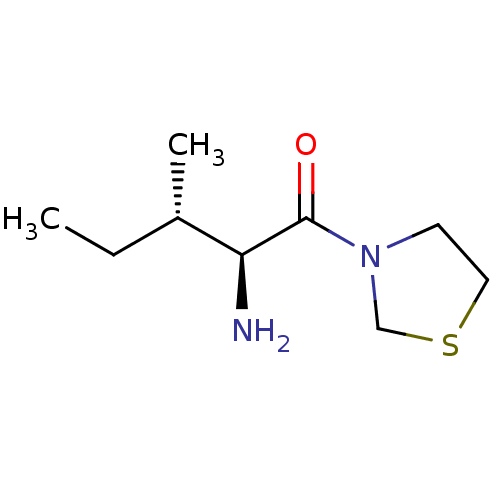
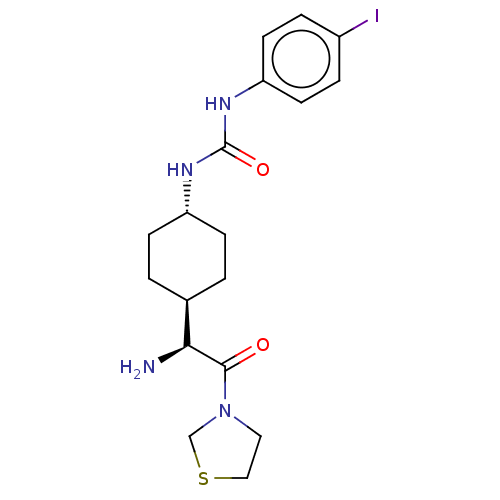
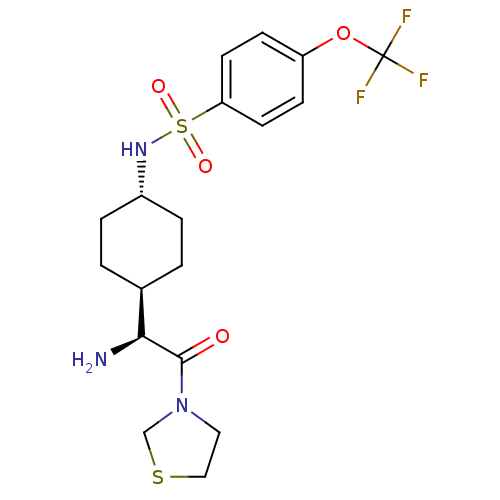
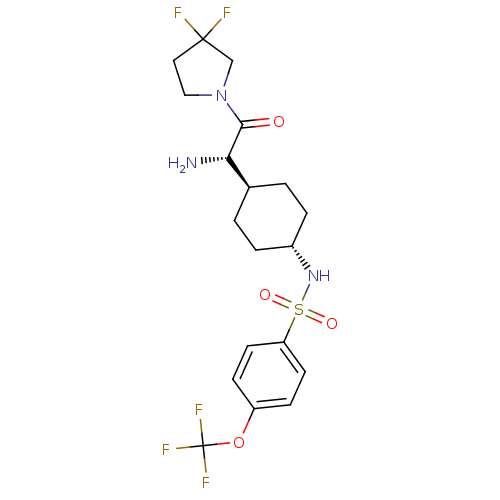
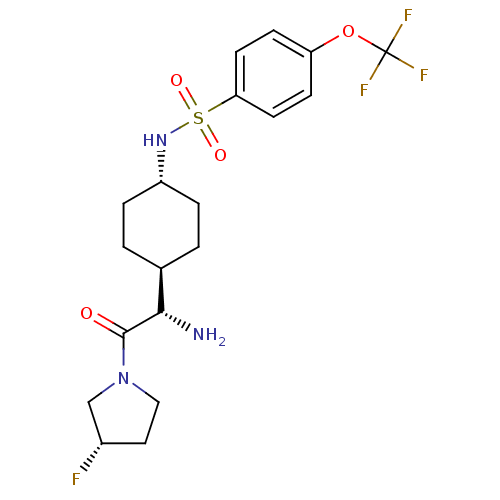
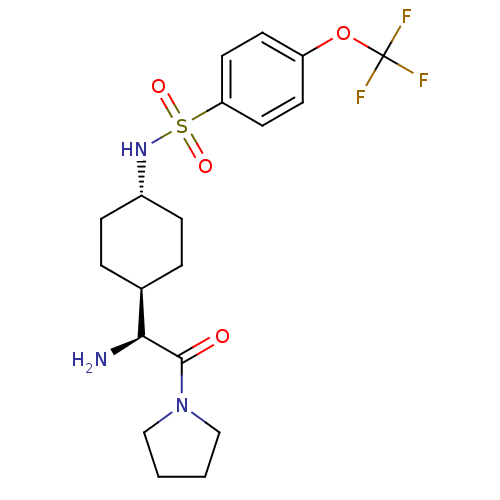
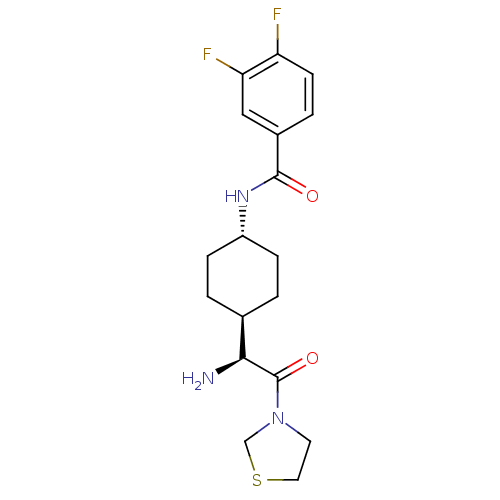
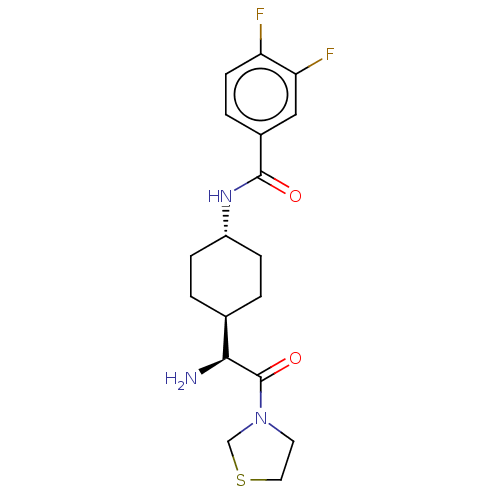
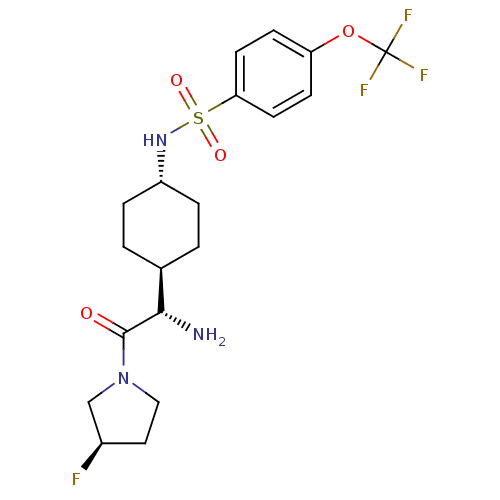
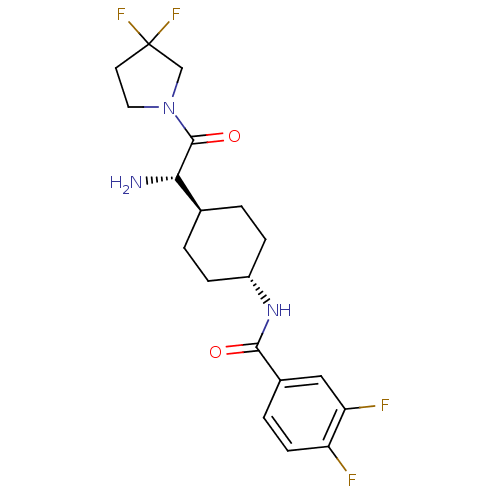
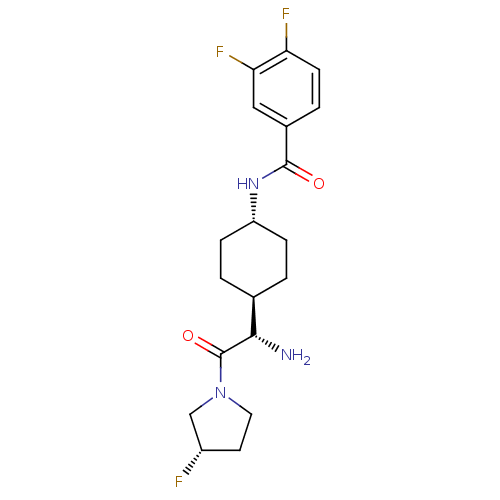
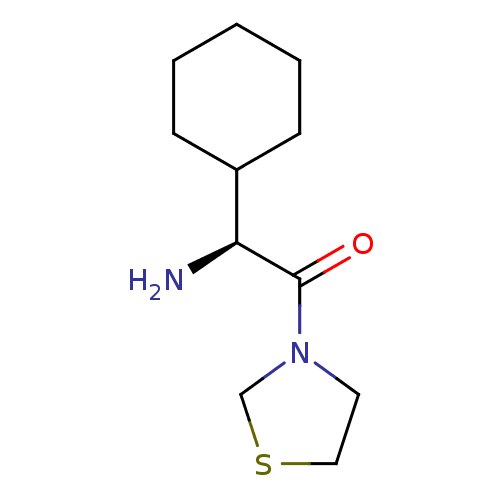
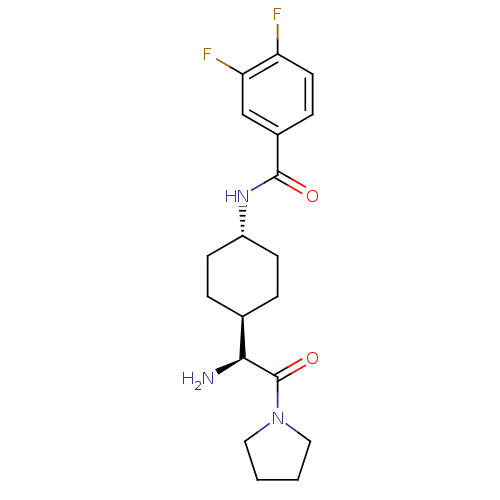
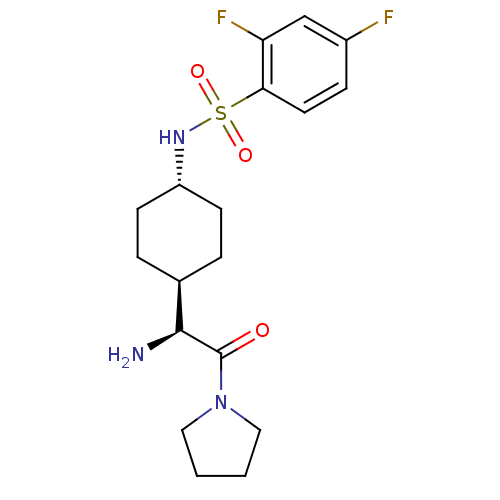
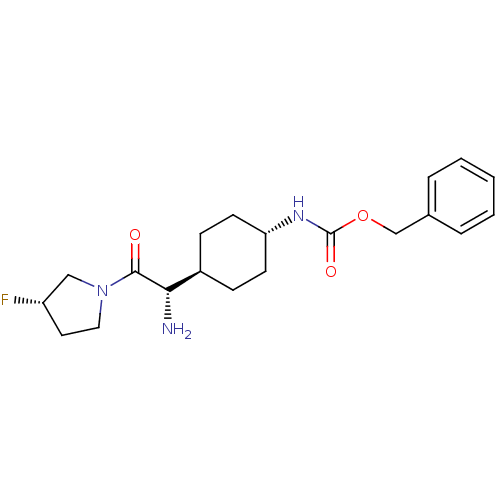
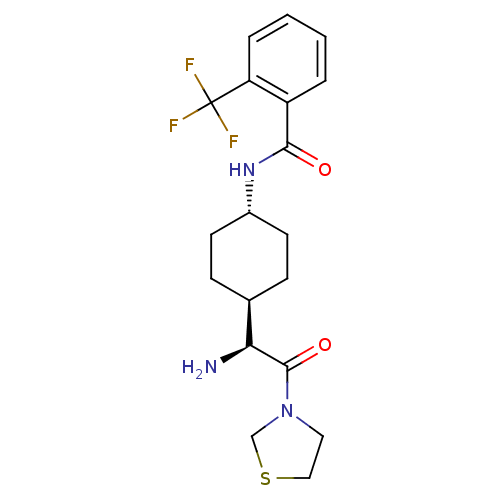
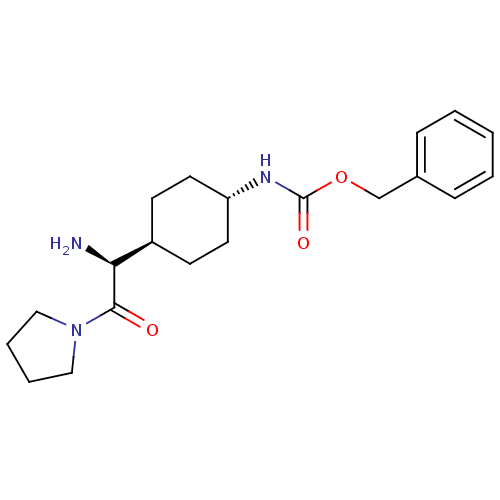
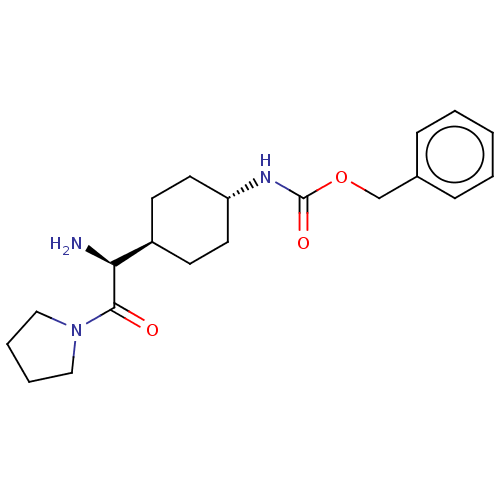
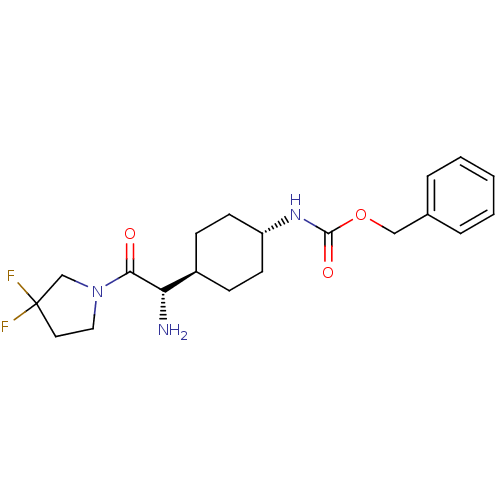
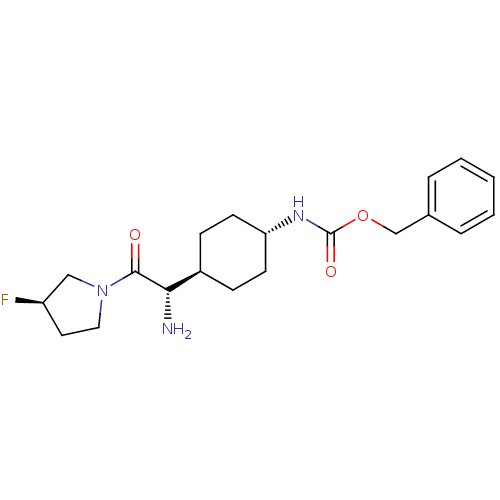
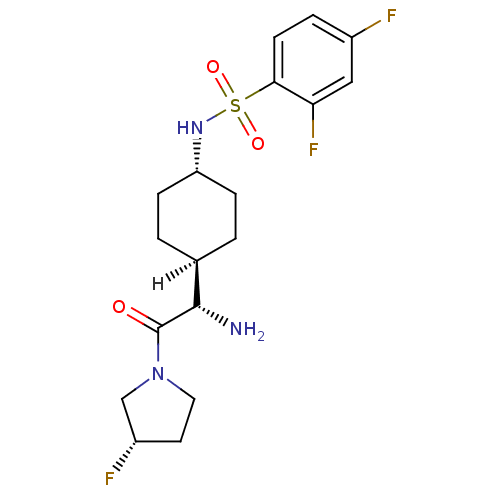
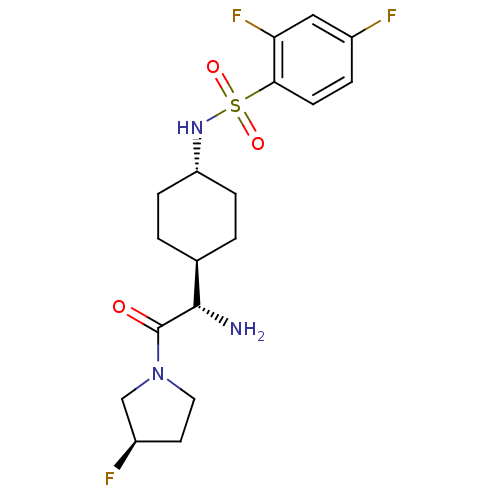
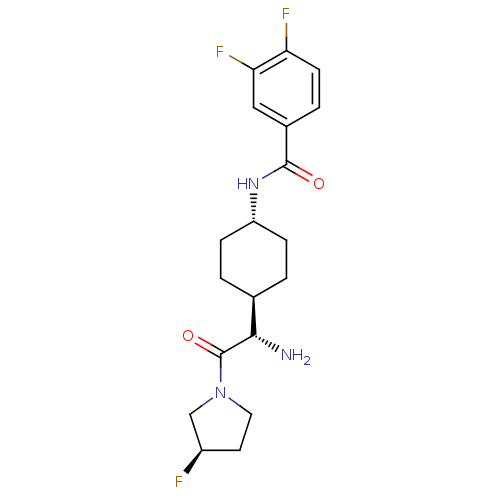
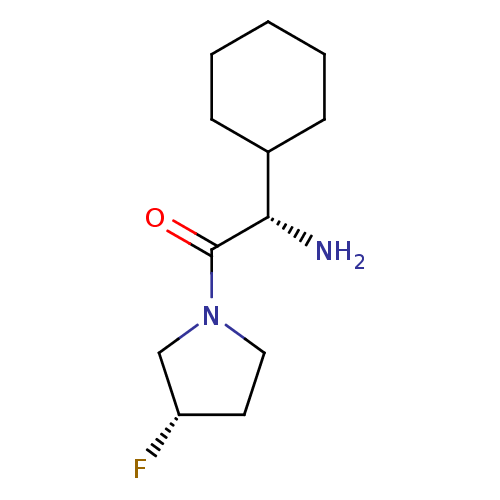
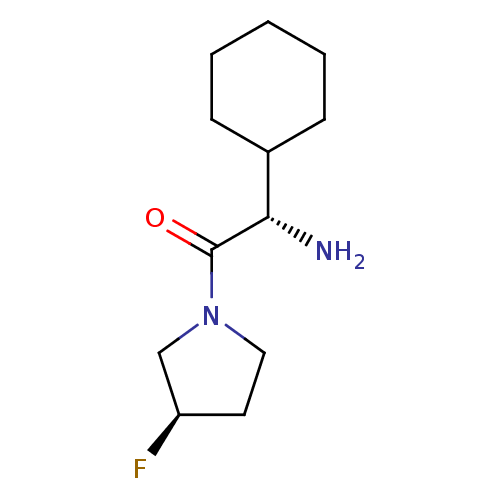
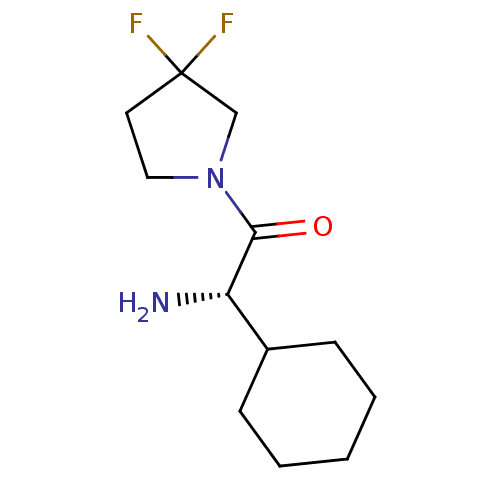
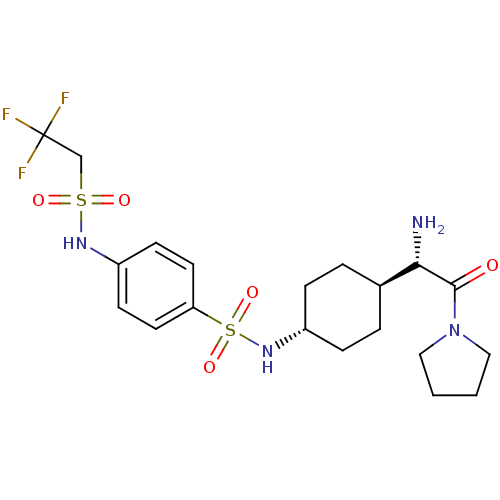
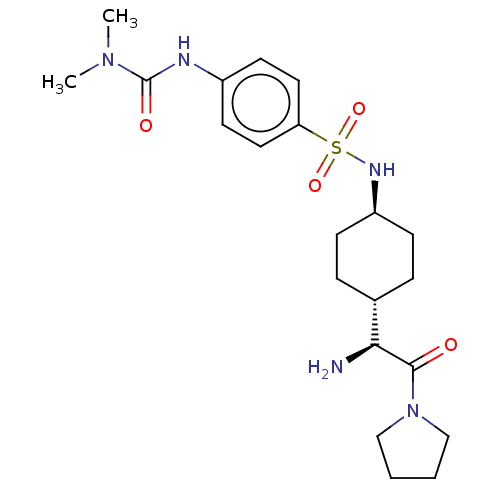
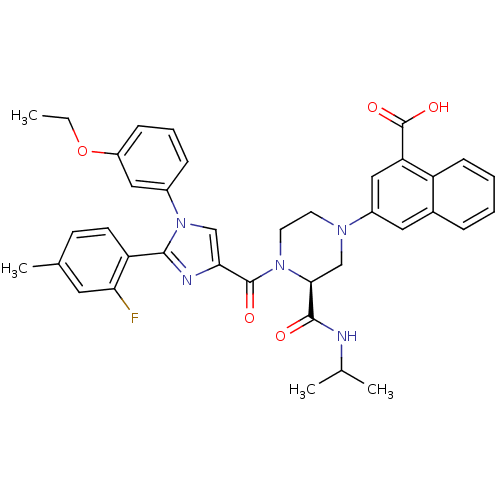
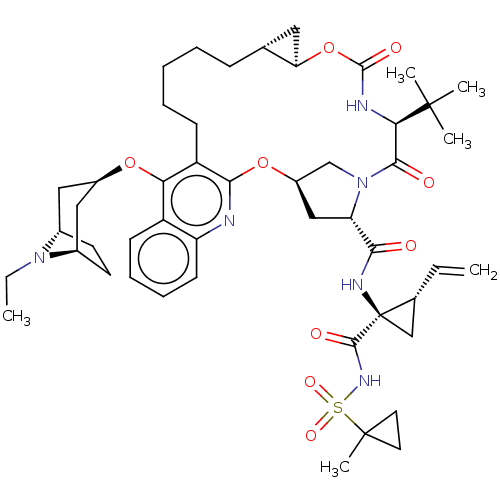
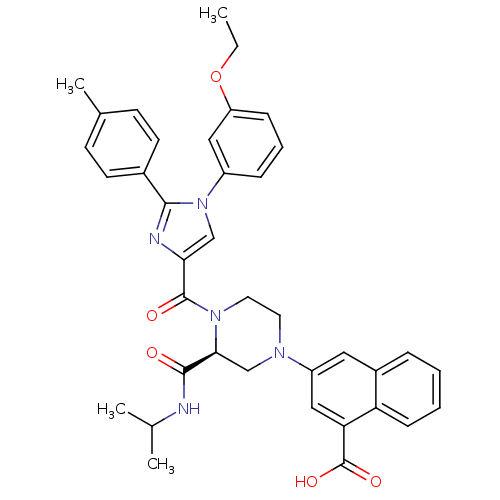
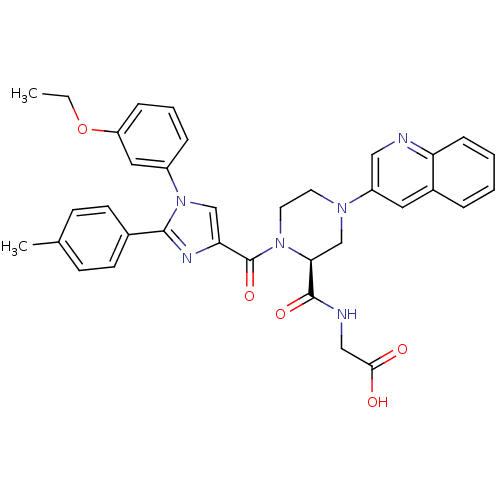
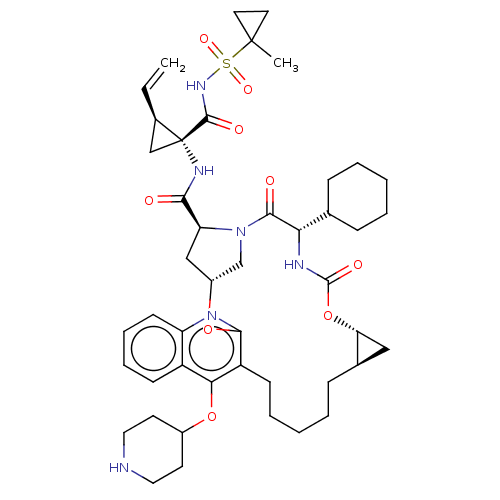
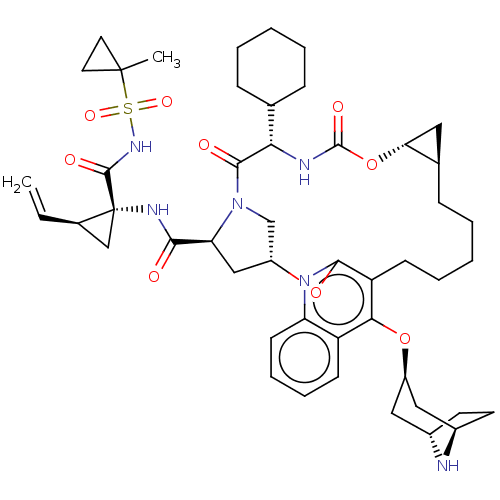
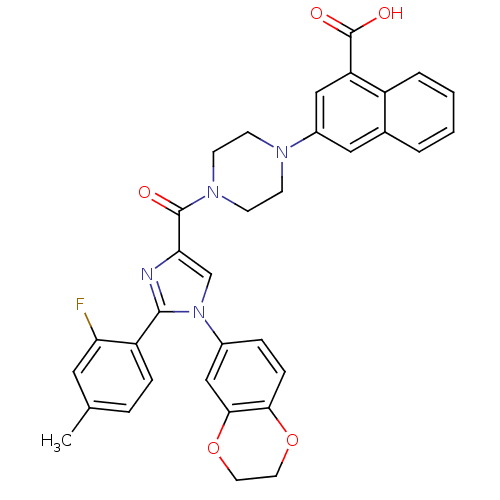
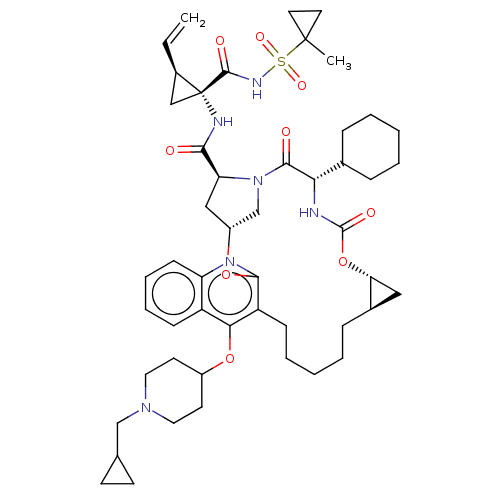
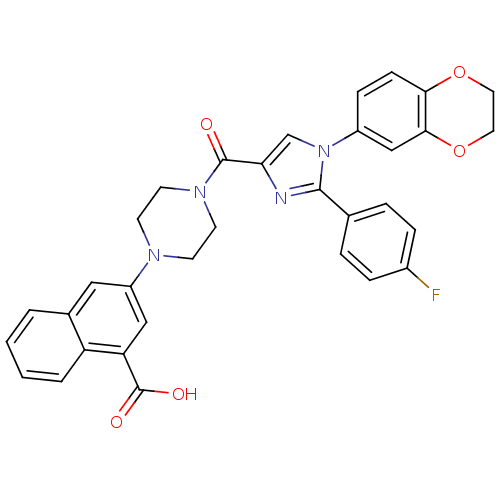
Affinity DataKi: 0.800nMAssay Description:Competitive reversible inhibition of DPP4 (unknown origin)More data for this Ligand-Target Pair
Affinity DataKi: 2nMAssay Description:Binding affinity for dipeptidyl-peptidase IVMore data for this Ligand-Target Pair
Affinity DataKi: 64nMAssay Description:Inhibitory concentration against dipeptidyl-peptidase IVMore data for this Ligand-Target Pair
Affinity DataKi: 125nMAssay Description:Inhibition of quiescent cell proline dipeptidase (QPP)More data for this Ligand-Target Pair
Affinity DataKi: 126nMAssay Description:Binding affinity for dipeptidyl-peptidase IVMore data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
Merck
Curated by ChEMBL
Merck
Curated by ChEMBL
Affinity DataKi: 1.90E+3nMAssay Description:Binding affinity for Potassium channel HERG Kv11.1More data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
Merck
Curated by ChEMBL
Merck
Curated by ChEMBL
Affinity DataKi: 2.80E+3nMAssay Description:Binding affinity of the compound towards human ERG potassium ion channel was determinedMore data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
Merck
Curated by ChEMBL
Merck
Curated by ChEMBL
Affinity DataKi: 2.80E+3nMAssay Description:Binding affinity for Potassium channel HERG Kv11.1More data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
Merck
Curated by ChEMBL
Merck
Curated by ChEMBL
Affinity DataKi: 5.30E+3nMAssay Description:Binding affinity of the compound towards human ERG potassium ion channel was determinedMore data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
Merck
Curated by ChEMBL
Merck
Curated by ChEMBL
Affinity DataKi: 5.50E+3nMAssay Description:Binding affinity of the compound towards human ERG potassium ion channel was determinedMore data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
Merck
Curated by ChEMBL
Merck
Curated by ChEMBL
Affinity DataKi: 5.70E+3nMAssay Description:Binding affinity of the compound towards human ERG potassium ion channel was determinedMore data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
Merck
Curated by ChEMBL
Merck
Curated by ChEMBL
Affinity DataKi: 5.70E+3nMAssay Description:Binding affinity for Potassium channel HERG Kv11.1More data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
Merck
Curated by ChEMBL
Merck
Curated by ChEMBL
Affinity DataKi: 5.90E+3nMAssay Description:Binding affinity of the compound towards human ERG potassium ion channel was determinedMore data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
Merck
Curated by ChEMBL
Merck
Curated by ChEMBL
Affinity DataKi: 5.90E+3nMAssay Description:Binding affinity for Potassium channel HERG Kv11.1More data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
Merck
Curated by ChEMBL
Merck
Curated by ChEMBL
Affinity DataKi: 6.00E+3nMAssay Description:Binding affinity of the compound towards human ERG potassium ion channel was determinedMore data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
Merck
Curated by ChEMBL
Merck
Curated by ChEMBL
Affinity DataKi: 1.60E+4nMAssay Description:Binding affinity of the compound towards human ERG potassium ion channel was determinedMore data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
Merck
Curated by ChEMBL
Merck
Curated by ChEMBL
Affinity DataKi: 1.70E+4nMAssay Description:Binding affinity for Potassium channel HERG Kv11.1More data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
Merck
Curated by ChEMBL
Merck
Curated by ChEMBL
Affinity DataKi: 1.90E+4nMAssay Description:Binding affinity of the compound towards human ERG potassium ion channel was determinedMore data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
Merck
Curated by ChEMBL
Merck
Curated by ChEMBL
Affinity DataKi: 2.20E+4nMAssay Description:Binding affinity for Potassium channel HERG Kv11.1More data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
Merck
Curated by ChEMBL
Merck
Curated by ChEMBL
Affinity DataKi: 2.80E+4nMAssay Description:Binding affinity of the compound towards human ERG potassium ion channel was determinedMore data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
Merck
Curated by ChEMBL
Merck
Curated by ChEMBL
Affinity DataKi: 2.90E+4nMAssay Description:Binding affinity of the compound towards human ERG potassium ion channel was determinedMore data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
Merck
Curated by ChEMBL
Merck
Curated by ChEMBL
Affinity DataKi: 3.50E+4nMAssay Description:Binding affinity for Potassium channel HERG Kv11.1More data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
Merck
Curated by ChEMBL
Merck
Curated by ChEMBL
Affinity DataKi: 3.50E+4nMAssay Description:Binding affinity of the compound towards human ERG potassium ion channel was determinedMore data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
Merck
Curated by ChEMBL
Merck
Curated by ChEMBL
Affinity DataKi: 3.70E+4nMAssay Description:Binding affinity of the compound towards human ERG potassium ion channel was determinedMore data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
Merck
Curated by ChEMBL
Merck
Curated by ChEMBL
Affinity DataKi: 3.90E+4nMAssay Description:Binding affinity of the compound towards human ERG potassium ion channel was determinedMore data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
Merck
Curated by ChEMBL
Merck
Curated by ChEMBL
Affinity DataKi: 4.00E+4nMAssay Description:Binding affinity of the compound towards human ERG potassium ion channel was determinedMore data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
Merck
Curated by ChEMBL
Merck
Curated by ChEMBL
Affinity DataKi: 4.00E+4nMAssay Description:Binding affinity for Potassium channel HERG Kv11.1More data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
Merck
Curated by ChEMBL
Merck
Curated by ChEMBL
Affinity DataKi: 4.20E+4nMAssay Description:Binding affinity of the compound towards human ERG potassium ion channel was determinedMore data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
Merck
Curated by ChEMBL
Merck
Curated by ChEMBL
Affinity DataKi: 4.30E+4nMAssay Description:Binding affinity of the compound towards human ERG potassium ion channel was determinedMore data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
Merck
Curated by ChEMBL
Merck
Curated by ChEMBL
Affinity DataKi: 4.90E+4nMAssay Description:Binding affinity of the compound towards human ERG potassium ion channel was determinedMore data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
Merck
Curated by ChEMBL
Merck
Curated by ChEMBL
Affinity DataKi: 5.20E+4nMAssay Description:Binding affinity of the compound towards human ERG potassium ion channel was determinedMore data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
Merck
Curated by ChEMBL
Merck
Curated by ChEMBL
Affinity DataKi: 7.30E+4nMAssay Description:Binding affinity of the compound towards human ERG potassium ion channel was determinedMore data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
Merck
Curated by ChEMBL
Merck
Curated by ChEMBL
Affinity DataKi: >9.00E+4nMAssay Description:Binding affinity of the compound towards human ERG potassium ion channel was determinedMore data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
Merck
Curated by ChEMBL
Merck
Curated by ChEMBL
Affinity DataKi: >9.00E+4nMAssay Description:Binding affinity of the compound towards human ERG potassium ion channel was determinedMore data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
Merck
Curated by ChEMBL
Merck
Curated by ChEMBL
Affinity DataKi: >9.00E+4nMAssay Description:Binding affinity of the compound towards human ERG potassium ion channel was determinedMore data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
Merck
Curated by ChEMBL
Merck
Curated by ChEMBL
Affinity DataKi: >1.00E+5nMAssay Description:Binding affinity for Potassium channel HERG Kv11.1More data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
Merck
Curated by ChEMBL
Merck
Curated by ChEMBL
Affinity DataKi: >1.00E+5nMAssay Description:Binding affinity for Potassium channel HERG Kv11.1More data for this Ligand-Target Pair
Affinity DataIC50: 0.0100nMAssay Description:Inhibition of human CCK1 receptorMore data for this Ligand-Target Pair
Affinity DataIC50: 0.0100nMAssay Description:Inhibition of Hepatitis C virus genotype 1b NS3/4A protease R155K mutant assessed as substrate cleavage using Ac-C(Eu)DDMEEAbu(COO)ASK(QSY7)-amide as...More data for this Ligand-Target Pair
Affinity DataIC50: 0.0160nMAssay Description:Inhibition of human CCK1 receptorMore data for this Ligand-Target Pair
Affinity DataIC50: 0.0180nMAssay Description:Inhibition of human CCK1 receptorMore data for this Ligand-Target Pair
Affinity DataIC50: 0.0200nMAssay Description:Inhibition of Hepatitis C virus genotype 1b NS3/4A protease R155K mutant assessed as substrate cleavage using Ac-C(Eu)DDMEEAbu(COO)ASK(QSY7)-amide as...More data for this Ligand-Target Pair
Affinity DataIC50: 0.0200nMAssay Description:Inhibition of Hepatitis C virus genotype 1b NS3/4A protease R155K mutant assessed as substrate cleavage using Ac-C(Eu)DDMEEAbu(COO)ASK(QSY7)-amide as...More data for this Ligand-Target Pair
Affinity DataIC50: 0.0200nMAssay Description:Inhibition of Hepatitis C virus genotype 1b NS3/4A protease R155K mutant assessed as substrate cleavage using Ac-C(Eu)DDMEEAbu(COO)ASK(QSY7)-amide as...More data for this Ligand-Target Pair
Affinity DataIC50: 0.0300nMAssay Description:Inhibition of human CCK1 receptorMore data for this Ligand-Target Pair
Affinity DataIC50: 0.0300nMAssay Description:Inhibition of Hepatitis C virus genotype 1b NS3/4A protease R155K mutant assessed as substrate cleavage using Ac-C(Eu)DDMEEAbu(COO)ASK(QSY7)-amide as...More data for this Ligand-Target Pair
Affinity DataIC50: 0.0300nMAssay Description:Inhibition of human CCK1 receptorMore data for this Ligand-Target Pair
Affinity DataIC50: 0.0340nMAssay Description:Displacement of [I125]-CCK8 from human CCK1 receptor expressed in CHO Flip cells after 2 hrs by scintillation countingMore data for this Ligand-Target Pair
Affinity DataIC50: 0.0390nMAssay Description:Inhibition of human CCK1 receptorMore data for this Ligand-Target Pair
Affinity DataIC50: 0.0400nMAssay Description:Inhibition of human CCK1 receptorMore data for this Ligand-Target Pair