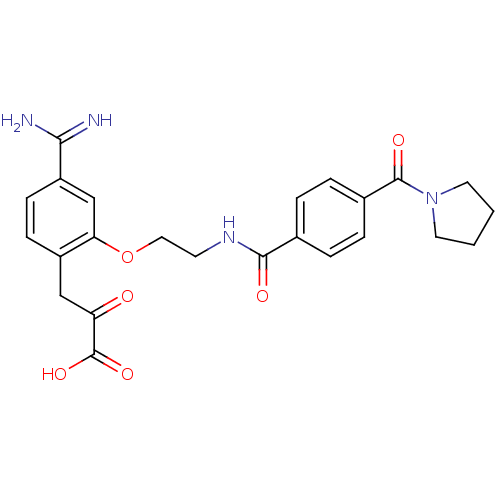
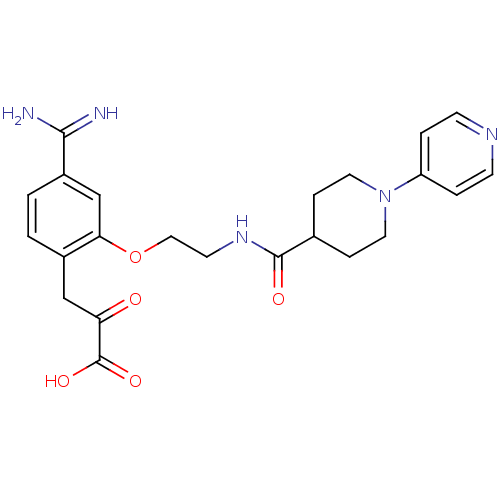
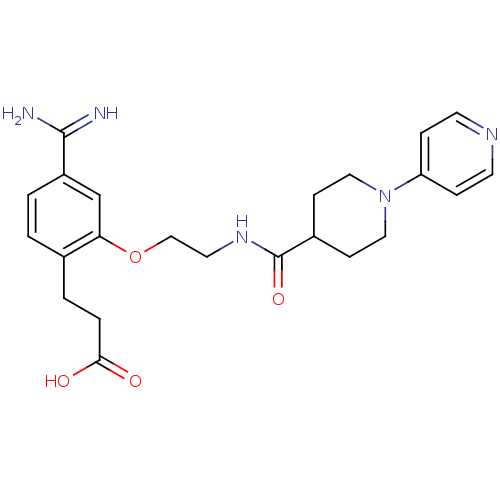
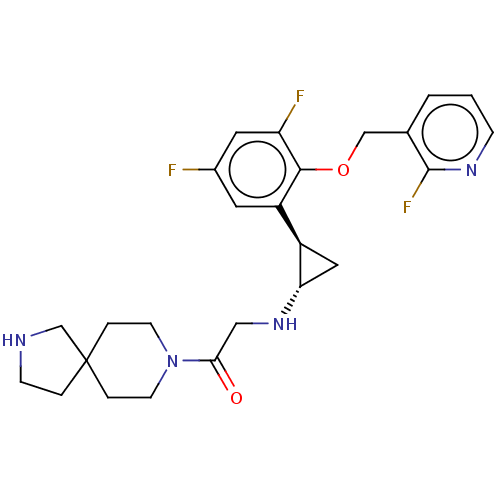
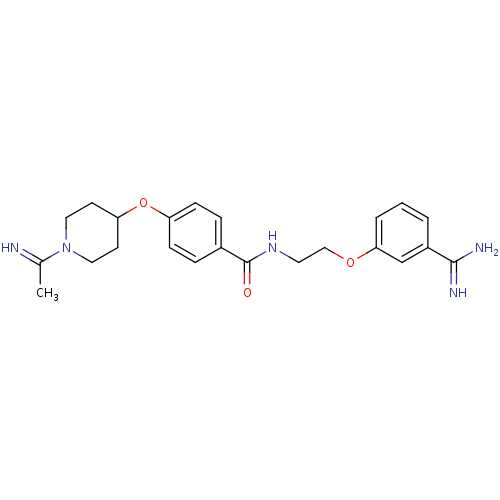
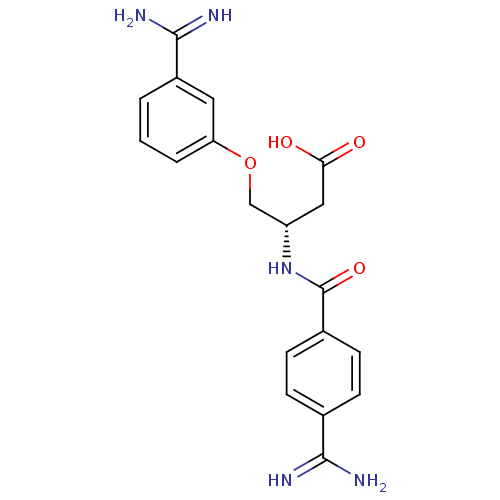
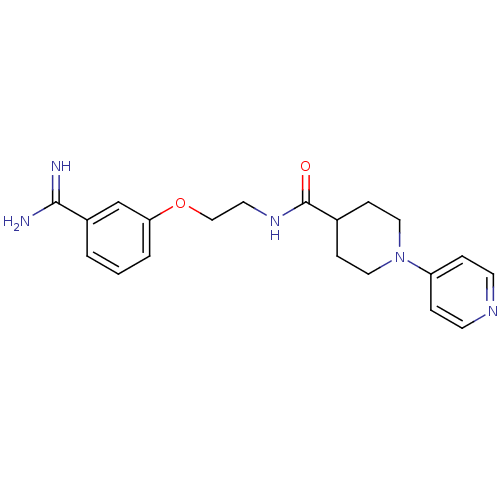
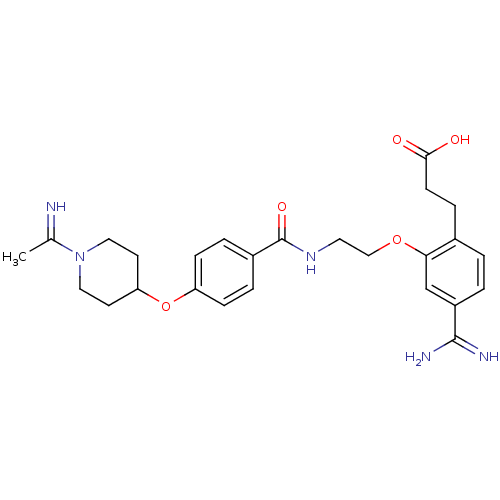
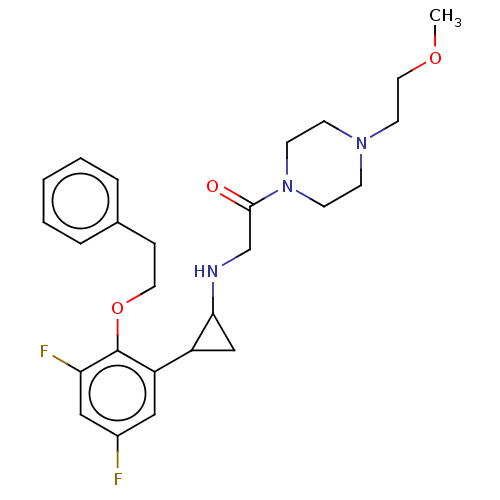
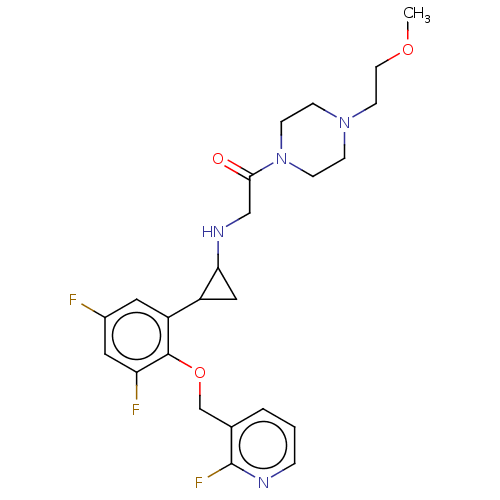
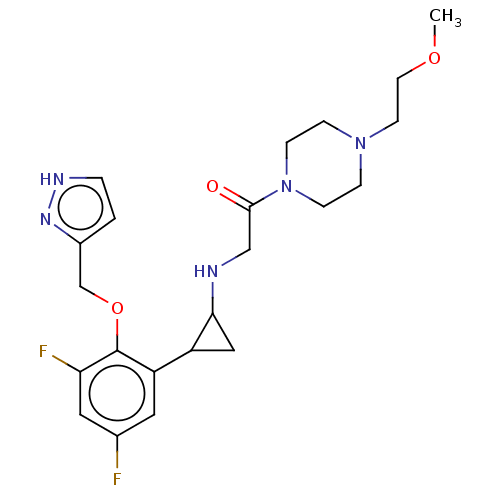
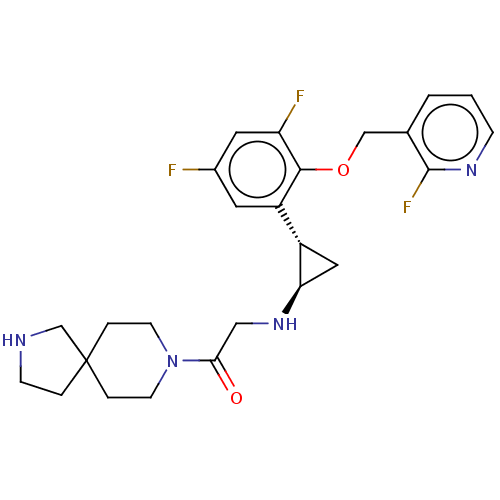
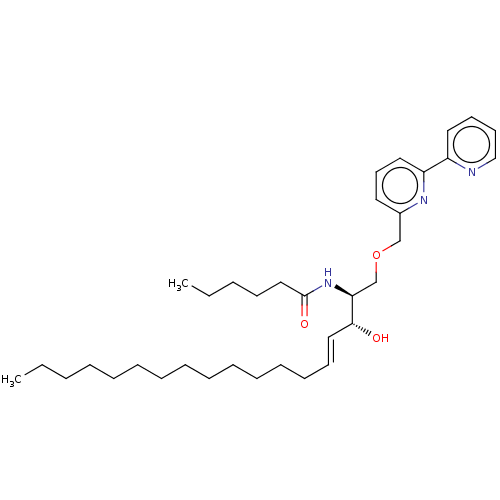
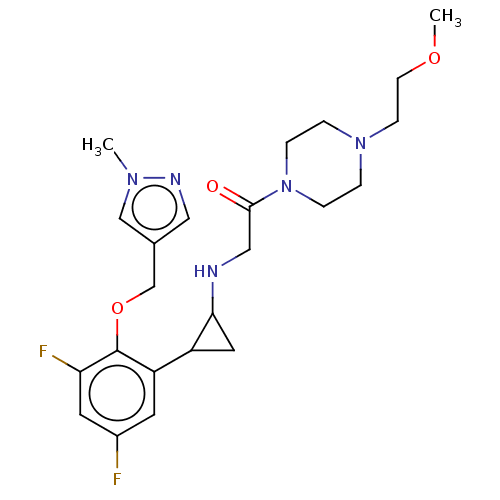
Affinity DataKi: 1.10nMAssay Description:Inhibition of human 11beta-HSD1 using variable cofactor NADPH concentration by Lineweaver burk plotMore data for this Ligand-Target Pair
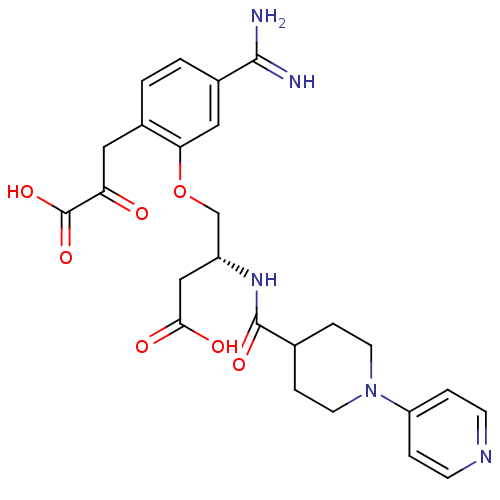
Affinity DataKi: 2.70nMAssay Description:Inhibition of human 11beta-HSD1 using variable substrate cortisol concentration by Lineweaver burk plotMore data for this Ligand-Target Pair
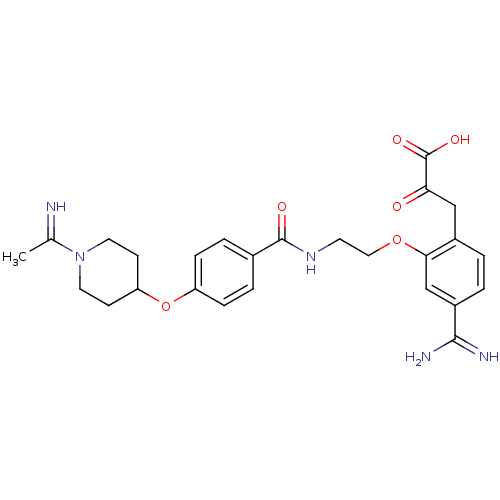
Affinity DataKi: 3nMAssay Description:Inhibitory activity against Coagulation factor X (fXa)More data for this Ligand-Target Pair
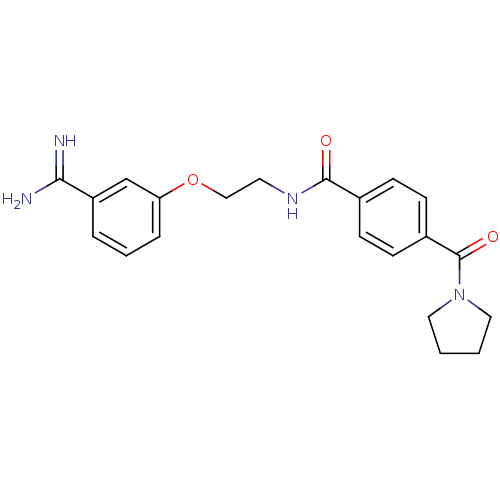
Affinity DataKi: 11nMAssay Description:Inhibitory activity against Coagulation factor X (fXa)More data for this Ligand-Target Pair
Affinity DataKi: 18nMAssay Description:Inhibitory activity against Coagulation factor X (fXa)More data for this Ligand-Target Pair
Affinity DataKi: 20nMAssay Description:Inhibitory activity against Coagulation factor X (fXa)More data for this Ligand-Target Pair
Affinity DataKi: 20nMAssay Description:Inhibitory activity against Coagulation factor X (fXa)More data for this Ligand-Target Pair
Affinity DataKi: 21nMAssay Description:Inhibitory activity against Coagulation factor X (fXa)More data for this Ligand-Target Pair
Affinity DataKi: 26nMAssay Description:Inhibitory activity against Coagulation factor X (fXa)More data for this Ligand-Target Pair
Affinity DataKi: 28nMAssay Description:Inhibitory activity against Coagulation factor X (fXa)More data for this Ligand-Target Pair
Affinity DataKi: 43nMAssay Description:Inhibitory activity against Coagulation factor X (fXa)More data for this Ligand-Target Pair
TargetLysine-specific histone demethylase 1A(Homo sapiens (Human))
Riken Center For Sustainable Resource Science
Curated by ChEMBL
Riken Center For Sustainable Resource Science
Curated by ChEMBL
Affinity DataKi: 80nMAssay Description:Inhibition of human LSD1 assessed as inhibitory constant using Histone H3(1-20)K4me2 peptide as substrate incubated for 10 mins followed by substrate...More data for this Ligand-Target Pair
Affinity DataKi: 170nMAssay Description:Inhibitory activity against Coagulation factor X (fXa)More data for this Ligand-Target Pair
Affinity DataKi: 170nMAssay Description:Inhibitory activity against Coagulation factor X (fXa)More data for this Ligand-Target Pair
TargetLysine-specific histone demethylase 1A(Homo sapiens (Human))
Riken Center For Sustainable Resource Science
Curated by ChEMBL
Riken Center For Sustainable Resource Science
Curated by ChEMBL
Affinity DataKi: 190nMAssay Description:Inhibition of human LSD1 assessed as inhibitory constant using Histone H3(1-20)K4me2 peptide as substrate incubated for 10 mins followed by substrate...More data for this Ligand-Target Pair
Affinity DataKi: 220nMAssay Description:Inhibitory activity against Coagulation factor X (fXa)More data for this Ligand-Target Pair
TargetLysine-specific histone demethylase 1A(Homo sapiens (Human))
Riken Center For Sustainable Resource Science
Curated by ChEMBL
Riken Center For Sustainable Resource Science
Curated by ChEMBL
Affinity DataKi: 220nMAssay Description:Inhibition of human LSD1 assessed as inhibitory constant using Histone H3(1-20)K4me2 peptide as substrate incubated for 10 mins followed by substrate...More data for this Ligand-Target Pair
Affinity DataKi: 290nMAssay Description:Inhibitory activity against Coagulation factor X (fXa)More data for this Ligand-Target Pair
Affinity DataKi: 330nMAssay Description:Inhibitory activity against Coagulation factor X (fXa)More data for this Ligand-Target Pair
TargetLysine-specific histone demethylase 1A(Homo sapiens (Human))
Riken Center For Sustainable Resource Science
Curated by ChEMBL
Riken Center For Sustainable Resource Science
Curated by ChEMBL
Affinity DataKi: 470nMAssay Description:Inhibition of human LSD1 assessed as inhibitory constant using Histone H3(1-20)K4me2 peptide as substrate incubated for 10 mins followed by substrate...More data for this Ligand-Target Pair
TargetLysine-specific histone demethylase 1A(Homo sapiens (Human))
Riken Center For Sustainable Resource Science
Curated by ChEMBL
Riken Center For Sustainable Resource Science
Curated by ChEMBL
Affinity DataKi: 720nMAssay Description:Inhibition of human LSD1 assessed as inhibitory constant using Histone H3(1-20)K4me2 peptide as substrate incubated for 10 mins followed by substrate...More data for this Ligand-Target Pair
TargetLysine-specific histone demethylase 1A(Homo sapiens (Human))
Riken Center For Sustainable Resource Science
Curated by ChEMBL
Riken Center For Sustainable Resource Science
Curated by ChEMBL
Affinity DataKi: 730nMAssay Description:Inhibition of human LSD1 assessed as inhibitory constant using Histone H3(1-20)K4me2 peptide as substrate incubated for 10 mins followed by substrate...More data for this Ligand-Target Pair
TargetLysine-specific histone demethylase 1A(Homo sapiens (Human))
Riken Center For Sustainable Resource Science
Curated by ChEMBL
Riken Center For Sustainable Resource Science
Curated by ChEMBL
Affinity DataKi: 750nMAssay Description:Inhibition of human LSD1 assessed as inhibitory constant using Histone H3(1-20)K4me2 peptide as substrate incubated for 10 mins followed by substrate...More data for this Ligand-Target Pair
Affinity DataKi: 1.30E+3nM ΔG°: -34.9kJ/mole IC50: 800nMpH: 7.5 T: 2°CAssay Description:Briefly, Bc-SMase (50 ng/ml) was treated at 37 °C for 60 min with various compounds that were solubilized in dimethylacetoamide. The reaction mix...More data for this Ligand-Target Pair
TargetLysine-specific histone demethylase 1A(Homo sapiens (Human))
Riken Center For Sustainable Resource Science
Curated by ChEMBL
Riken Center For Sustainable Resource Science
Curated by ChEMBL
Affinity DataKi: 1.30E+3nMAssay Description:Inhibition of human LSD1 assessed as inhibitory constant using Histone H3(1-20)K4me2 peptide as substrate incubated for 10 mins followed by substrate...More data for this Ligand-Target Pair
TargetLysine-specific histone demethylase 1A(Homo sapiens (Human))
Riken Center For Sustainable Resource Science
Curated by ChEMBL
Riken Center For Sustainable Resource Science
Curated by ChEMBL
Affinity DataKi: 1.30E+3nMAssay Description:Inhibition of human LSD1 assessed as inhibitory constant using Histone H3(1-20)K4me2 peptide as substrate incubated for 10 mins followed by substrate...More data for this Ligand-Target Pair
TargetLysine-specific histone demethylase 1A(Homo sapiens (Human))
Riken Center For Sustainable Resource Science
Curated by ChEMBL
Riken Center For Sustainable Resource Science
Curated by ChEMBL
Affinity DataKi: 1.40E+3nMAssay Description:Inhibition of human LSD1 assessed as inhibitory constant using Histone H3(1-20)K4me2 peptide as substrate incubated for 10 mins followed by substrate...More data for this Ligand-Target Pair
Affinity DataKi: 2.80E+3nM ΔG°: -33.0kJ/mole IC50: 900nMpH: 7.5 T: 2°CAssay Description:Briefly, Bc-SMase (50 ng/ml) was treated at 37 °C for 60 min with various compounds that were solubilized in dimethylacetoamide. The reaction mix...More data for this Ligand-Target Pair
Affinity DataKi: 2.80E+3nMAssay Description:Inhibitory activity against Coagulation factor X (fXa)More data for this Ligand-Target Pair
TargetLysine-specific histone demethylase 1A(Homo sapiens (Human))
Riken Center For Sustainable Resource Science
Curated by ChEMBL
Riken Center For Sustainable Resource Science
Curated by ChEMBL
Affinity DataKi: 2.80E+3nMAssay Description:Inhibition of human LSD1 assessed as inhibitory constant using Histone H3(1-20)K4me2 peptide as substrate incubated for 10 mins followed by substrate...More data for this Ligand-Target Pair
TargetLysine-specific histone demethylase 1A(Homo sapiens (Human))
Riken Center For Sustainable Resource Science
Curated by ChEMBL
Riken Center For Sustainable Resource Science
Curated by ChEMBL
Affinity DataKi: 2.90E+3nMAssay Description:Inhibition of human LSD1 assessed as inhibitory constant using Histone H3(1-20)K4me2 peptide as substrate incubated for 10 mins followed by substrate...More data for this Ligand-Target Pair
TargetLysine-specific histone demethylase 1A(Homo sapiens (Human))
Riken Center For Sustainable Resource Science
Curated by ChEMBL
Riken Center For Sustainable Resource Science
Curated by ChEMBL
Affinity DataKi: 4.50E+3nMAssay Description:Inhibition of human LSD1 assessed as inhibitory constant using Histone H3(1-20)K4me2 peptide as substrate incubated for 10 mins followed by substrate...More data for this Ligand-Target Pair
Affinity DataKi: 5.20E+3nM ΔG°: -31.4kJ/mole IC50: 1.20E+3nMpH: 7.5 T: 2°CAssay Description:Briefly, Bc-SMase (50 ng/ml) was treated at 37 °C for 60 min with various compounds that were solubilized in dimethylacetoamide. The reaction mix...More data for this Ligand-Target Pair
TargetLysine-specific histone demethylase 1A(Homo sapiens (Human))
Riken Center For Sustainable Resource Science
Curated by ChEMBL
Riken Center For Sustainable Resource Science
Curated by ChEMBL
Affinity DataKi: 6.00E+3nMAssay Description:Inhibition of human LSD1 assessed as inhibitory constant using Histone H3(1-20)K4me2 peptide as substrate incubated for 10 mins followed by substrate...More data for this Ligand-Target Pair
TargetLysine-specific histone demethylase 1A(Homo sapiens (Human))
Riken Center For Sustainable Resource Science
Curated by ChEMBL
Riken Center For Sustainable Resource Science
Curated by ChEMBL
Affinity DataKi: 1.00E+4nMAssay Description:Inhibition of human LSD1 assessed as inhibitory constant using Histone H3(1-20)K4me2 peptide as substrate incubated for 10 mins followed by substrate...More data for this Ligand-Target Pair
Affinity DataKi: 2.40E+4nMAssay Description:Inhibitory activity against thrombinMore data for this Ligand-Target Pair
Affinity DataKi: 3.80E+4nMAssay Description:Inhibitory activity against thrombinMore data for this Ligand-Target Pair
Affinity DataKi: 5.30E+4nMAssay Description:Inhibitory activity against thrombinMore data for this Ligand-Target Pair
Affinity DataKi: 1.10E+5nMAssay Description:Inhibitory activity against thrombinMore data for this Ligand-Target Pair
Affinity DataKi: 1.40E+5nMAssay Description:Inhibitory activity against thrombinMore data for this Ligand-Target Pair
TargetAmine oxidase [flavin-containing] B(Homo sapiens (Human))
Riken Center For Sustainable Resource Science
Curated by ChEMBL
Riken Center For Sustainable Resource Science
Curated by ChEMBL
Affinity DataKi: >2.50E+5nMAssay Description:Inhibition of MAO-B (unknown origin) using tyramine as substrate and measured after 10 mins by peroxidase-coupled reaction methodMore data for this Ligand-Target Pair
TargetAmine oxidase [flavin-containing] B(Homo sapiens (Human))
Riken Center For Sustainable Resource Science
Curated by ChEMBL
Riken Center For Sustainable Resource Science
Curated by ChEMBL
Affinity DataKi: >2.50E+5nMAssay Description:Inhibition of MAO-B (unknown origin) using tyramine as substrate and measured after 10 mins by peroxidase-coupled reaction methodMore data for this Ligand-Target Pair
TargetAmine oxidase [flavin-containing] A(Homo sapiens (Human))
Riken Center For Sustainable Resource Science
Curated by ChEMBL
Riken Center For Sustainable Resource Science
Curated by ChEMBL
Affinity DataKi: >2.50E+5nMAssay Description:Inhibition of MAO-A (unknown origin) using tyramine as substrate and measured after 10 mins by peroxidase-coupled reaction methodMore data for this Ligand-Target Pair
TargetAmine oxidase [flavin-containing] A(Homo sapiens (Human))
Riken Center For Sustainable Resource Science
Curated by ChEMBL
Riken Center For Sustainable Resource Science
Curated by ChEMBL
Affinity DataKi: >2.50E+5nMAssay Description:Inhibition of MAO-A (unknown origin) using tyramine as substrate and measured after 10 mins by peroxidase-coupled reaction methodMore data for this Ligand-Target Pair
Affinity DataKi: 4.40E+5nMAssay Description:Inhibitory activity against thrombinMore data for this Ligand-Target Pair
Affinity DataKi: 5.40E+5nMAssay Description:Inhibitory activity against thrombinMore data for this Ligand-Target Pair
Affinity DataKi: 6.80E+5nMAssay Description:Inhibitory activity against thrombinMore data for this Ligand-Target Pair
Affinity DataKi: 7.10E+5nMAssay Description:Inhibitory activity against thrombinMore data for this Ligand-Target Pair
Affinity DataKi: 8.00E+5nMAssay Description:Inhibitory activity against thrombinMore data for this Ligand-Target Pair
Affinity DataKi: 1.00E+6nMAssay Description:Inhibitory activity against thrombinMore data for this Ligand-Target Pair

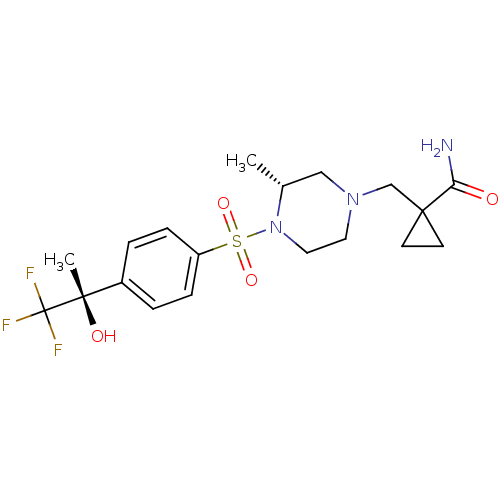
 3D Structure (crystal)
3D Structure (crystal)