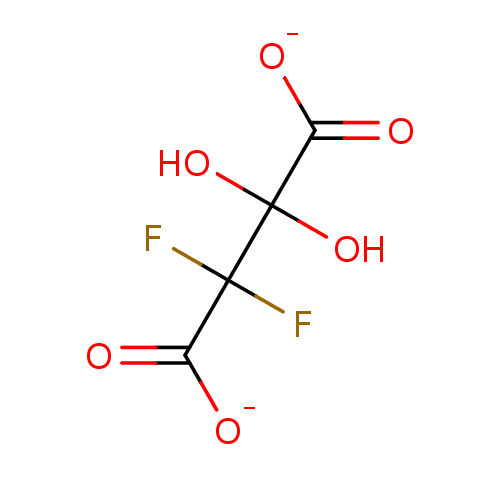
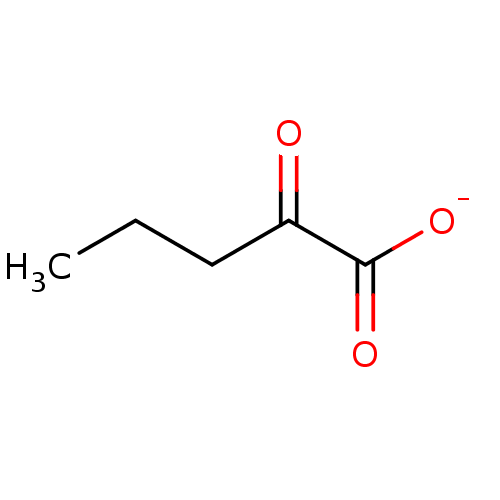
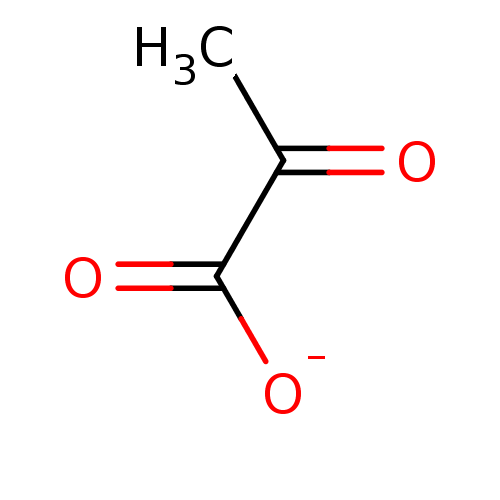
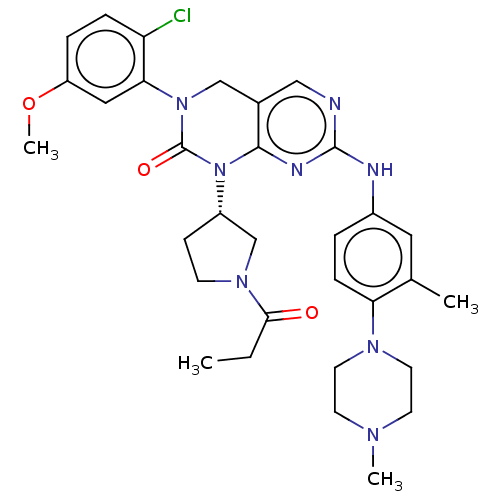
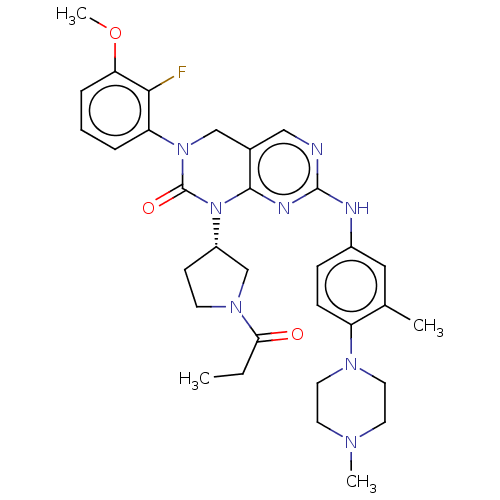
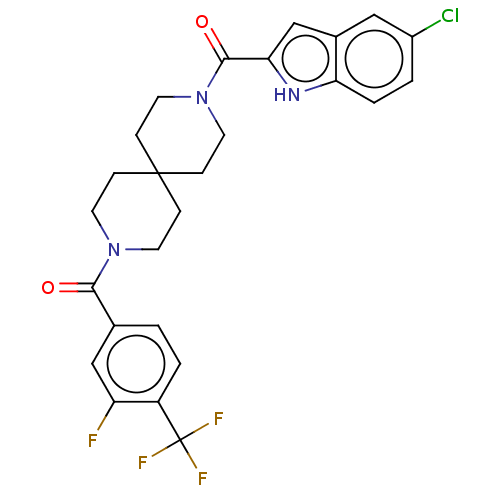
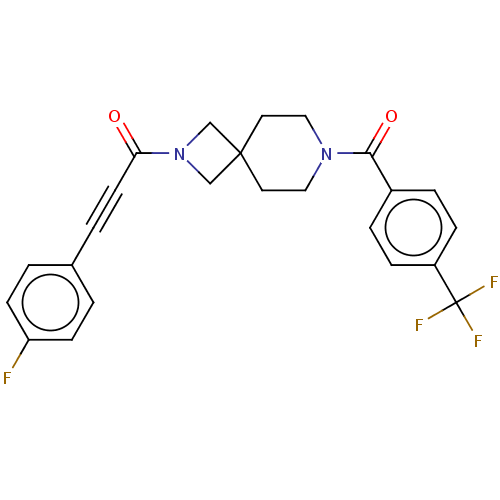
TargetEnoyl-[acyl-carrier-protein] reductase [NADH](Yersinia pestis (Enterobacteria))
University Of Wurzburg
University Of Wurzburg
Affinity DataKi: 100nM ΔG°: -40.0kJ/molepH: 8.0 T: 2°CAssay Description:Steady-state kinetics were performed on a Cary 100 Bio (Varian) spectrometer at 25 °C using 30 mM PIPES, 150 mM NaCl, and 1.0 mM EDTA (pH 8.0) as...More data for this Ligand-Target Pair
TargetEnoyl-[acyl-carrier-protein] reductase [NADH](Yersinia pestis (Enterobacteria))
University Of Wurzburg
University Of Wurzburg
Affinity DataKi: 200nM ΔG°: -38.2kJ/molepH: 8.0 T: 2°CAssay Description:Steady-state kinetics were performed on a Cary 100 Bio (Varian) spectrometer at 25 °C using 30 mM PIPES, 150 mM NaCl, and 1.0 mM EDTA (pH 8.0) as...More data for this Ligand-Target Pair
TargetEnoyl-[acyl-carrier-protein] reductase [NADH] [T276S](Yersinia pestis (Enterobacteria))
University Of Wurzburg
University Of Wurzburg
Affinity DataKi: 500nM ΔG°: -36.0kJ/molepH: 8.0 T: 2°CAssay Description:Steady-state kinetics were performed on a Cary 100 Bio (Varian) spectrometer at 25 °C using 30 mM PIPES, 150 mM NaCl, and 1.0 mM EDTA (pH 8.0) as...More data for this Ligand-Target Pair
Target11-beta-hydroxysteroid dehydrogenase 1(Mus musculus (mouse))
Chinese Academy Of Sciences
Curated by ChEMBL
Chinese Academy Of Sciences
Curated by ChEMBL
Affinity DataKi: 1.00E+3nMAssay Description:Inhibition of mouse liver microsome 11betaHSD1 reductase activity expressed in HEK293 cells by scintillation proximity assayMore data for this Ligand-Target Pair
TargetEnoyl-[acyl-carrier-protein] reductase [NADH] [T276S](Yersinia pestis (Enterobacteria))
University Of Wurzburg
University Of Wurzburg
Affinity DataKi: 3.00E+3nM ΔG°: -31.5kJ/molepH: 8.0 T: 2°CAssay Description:Steady-state kinetics were performed on a Cary 100 Bio (Varian) spectrometer at 25 °C using 30 mM PIPES, 150 mM NaCl, and 1.0 mM EDTA (pH 8.0) as...More data for this Ligand-Target Pair
TargetIntestinal-type alkaline phosphatase(Mus musculus)
Sanford-Burnham Medical Research Institute
Curated by ChEMBL
Sanford-Burnham Medical Research Institute
Curated by ChEMBL
Affinity DataKi: 3.20E+3nMAssay Description:Competitive inhibition of mouse duodenal-specific FLAG-tagged IAP expressed in African green monkey COS1 cells using p-nitrophenyl phosphate as subst...More data for this Ligand-Target Pair
TargetEnoyl-[acyl-carrier-protein] reductase [NADH] [T276S](Yersinia pestis (Enterobacteria))
University Of Wurzburg
University Of Wurzburg
Affinity DataKi: 4.00E+3nM ΔG°: -30.8kJ/molepH: 8.0 T: 2°CAssay Description:Steady-state kinetics were performed on a Cary 100 Bio (Varian) spectrometer at 25 °C using 30 mM PIPES, 150 mM NaCl, and 1.0 mM EDTA (pH 8.0) as...More data for this Ligand-Target Pair
TargetEnoyl-[acyl-carrier-protein] reductase [NADH](Yersinia pestis (Enterobacteria))
University Of Wurzburg
University Of Wurzburg
Affinity DataKi: 8.00E+3nM ΔG°: -29.1kJ/molepH: 8.0 T: 2°CAssay Description:Steady-state kinetics were performed on a Cary 100 Bio (Varian) spectrometer at 25 °C using 30 mM PIPES, 150 mM NaCl, and 1.0 mM EDTA (pH 8.0) as...More data for this Ligand-Target Pair
TargetEnoyl-[acyl-carrier-protein] reductase [NADH] [T276S](Yersinia pestis (Enterobacteria))
University Of Wurzburg
University Of Wurzburg
Affinity DataKi: 8.00E+3nM ΔG°: -29.1kJ/molepH: 8.0 T: 2°CAssay Description:Steady-state kinetics were performed on a Cary 100 Bio (Varian) spectrometer at 25 °C using 30 mM PIPES, 150 mM NaCl, and 1.0 mM EDTA (pH 8.0) as...More data for this Ligand-Target Pair
TargetEnoyl-[acyl-carrier-protein] reductase [NADH](Yersinia pestis (Enterobacteria))
University Of Wurzburg
University Of Wurzburg
Affinity DataKi: 1.60E+4nM ΔG°: -27.4kJ/molepH: 8.0 T: 2°CAssay Description:Steady-state kinetics were performed on a Cary 100 Bio (Varian) spectrometer at 25 °C using 30 mM PIPES, 150 mM NaCl, and 1.0 mM EDTA (pH 8.0) as...More data for this Ligand-Target Pair
TargetOxaloacetate decarboxylase(Pseudomonas aeruginosa (strain ATCC 15692 / PAO1 /...)
University Of Maryland Biotechnology Institute
University Of Maryland Biotechnology Institute
Affinity DataKi: 4.30E+4nMAssay Description:Competitive inhibition constant determined for PA4872 catalyzed oxaloacetate decarboxylation.More data for this Ligand-Target Pair
TargetOxaloacetate decarboxylase(Pseudomonas aeruginosa (strain ATCC 15692 / PAO1 /...)
University Of Maryland Biotechnology Institute
University Of Maryland Biotechnology Institute
Affinity DataKi: 4.50E+5nMAssay Description:Competitive inhibition constant determined for PA4872 catalyzed oxaloacetate decarboxylation.More data for this Ligand-Target Pair
TargetOxaloacetate decarboxylase(Pseudomonas aeruginosa (strain ATCC 15692 / PAO1 /...)
University Of Maryland Biotechnology Institute
University Of Maryland Biotechnology Institute
Affinity DataKi: 5.60E+5nMAssay Description:Competitive inhibition constant determined for PA4872 catalyzed oxaloacetate decarboxylation.More data for this Ligand-Target Pair
TargetOxaloacetate decarboxylase(Pseudomonas aeruginosa (strain ATCC 15692 / PAO1 /...)
University Of Maryland Biotechnology Institute
University Of Maryland Biotechnology Institute
Affinity DataKi: 1.09E+6nMAssay Description:Competitive inhibition constant determined for PA4872 catalyzed oxaloacetate decarboxylation.More data for this Ligand-Target Pair
TargetOxaloacetate decarboxylase(Pseudomonas aeruginosa (strain ATCC 15692 / PAO1 /...)
University Of Maryland Biotechnology Institute
University Of Maryland Biotechnology Institute
Affinity DataKi: 3.00E+6nMAssay Description:Competitive inhibition constant determined for PA4872 catalyzed oxaloacetate decarboxylation.More data for this Ligand-Target Pair
TargetOxaloacetate decarboxylase(Pseudomonas aeruginosa (strain ATCC 15692 / PAO1 /...)
University Of Maryland Biotechnology Institute
University Of Maryland Biotechnology Institute
Affinity DataKi: 3.20E+6nMAssay Description:Competitive inhibition constant determined for PA4872 catalyzed oxaloacetate decarboxylation.More data for this Ligand-Target Pair
TargetOxaloacetate decarboxylase(Pseudomonas aeruginosa (strain ATCC 15692 / PAO1 /...)
University Of Maryland Biotechnology Institute
University Of Maryland Biotechnology Institute
Affinity DataKi: 6.70E+6nMAssay Description:Competitive inhibition constant determined for PA4872 catalyzed oxaloacetate decarboxylation.More data for this Ligand-Target Pair
TargetOxaloacetate decarboxylase(Pseudomonas aeruginosa (strain ATCC 15692 / PAO1 /...)
University Of Maryland Biotechnology Institute
University Of Maryland Biotechnology Institute
Affinity DataKi: 7.20E+6nMAssay Description:Competitive inhibition constant determined for PA4872 catalyzed oxaloacetate decarboxylation.More data for this Ligand-Target Pair
TargetVascular endothelial growth factor receptor 2(Homo sapiens (Human))
Jinan University
Curated by ChEMBL
Jinan University
Curated by ChEMBL
Affinity DataIC50: 0.0350nMAssay Description:Inhibition of recombinant human full length KDR using poly(Glu, Tyr) as substrate by alphascreen assayMore data for this Ligand-Target Pair
TargetEpidermal growth factor receptor(Homo sapiens (Human))
Chinese Academy Of Sciences
Curated by ChEMBL
Chinese Academy Of Sciences
Curated by ChEMBL
Affinity DataIC50: 0.5nMAssay Description:Inhibition of recombinant human wild type GST-tagged EGFR cytoplasmic domain (668 to 1210 residues) expressed in baculovirus using tyr4 peptide as su...More data for this Ligand-Target Pair
Affinity DataIC50: 1nMAssay Description:Inhibition of recombinant Syk (unknown origin)More data for this Ligand-Target Pair
Affinity DataIC50: 1.30nMAssay Description:Inhibition of recombinant human full length MET using poly(Glu, Tyr) as substrate by alphascreen assayMore data for this Ligand-Target Pair
Affinity DataIC50: 1.60nMAssay Description:Inhibition of recombinant AXL (unknown origin) using poly (Glu,Tyr) 4:1 as substrate incubated for 60 mins in presence of ATP by ELISAMore data for this Ligand-Target Pair
Affinity DataIC50: 1.60nMAssay Description:Inhibition of recombinant Syk (unknown origin)More data for this Ligand-Target Pair
TargetMacrophage colony-stimulating factor 1 receptor(Homo sapiens (Human))
Chinese Academy Of Sciences
Curated by ChEMBL
Chinese Academy Of Sciences
Curated by ChEMBL
Affinity DataIC50: 2nMAssay Description:Inhibition of recombinant human His-tagged CSF1R cytoplasmic domain (538 to 910 residues) expressed in baculovirus using tyr4 peptide as substrate af...More data for this Ligand-Target Pair
Affinity DataIC50: 2nMAssay Description:Inhibition of human HDAC1More data for this Ligand-Target Pair
TargetMacrophage colony-stimulating factor 1 receptor(Homo sapiens (Human))
Chinese Academy Of Sciences
Curated by ChEMBL
Chinese Academy Of Sciences
Curated by ChEMBL
Affinity DataIC50: 2.10nMAssay Description:Inhibition of recombinant human His-tagged CSF1R cytoplasmic domain (538 to 910 residues) expressed in baculovirus using tyr4 peptide as substrate af...More data for this Ligand-Target Pair
Affinity DataIC50: 2.5nMAssay Description:Inhibition of recombinant Syk (unknown origin)More data for this Ligand-Target Pair
Affinity DataIC50: 2.90nMAssay Description:Inhibition of recombinant AXL (unknown origin) using poly (Glu,Tyr) 4:1 as substrate incubated for 60 mins in presence of ATP by ELISAMore data for this Ligand-Target Pair
TargetMacrophage colony-stimulating factor 1 receptor(Homo sapiens (Human))
Chinese Academy Of Sciences
Curated by ChEMBL
Chinese Academy Of Sciences
Curated by ChEMBL
Affinity DataIC50: 3nMAssay Description:Inhibition of recombinant human His-tagged CSF1R cytoplasmic domain (538 to 910 residues) expressed in baculovirus using tyr4 peptide as substrate af...More data for this Ligand-Target Pair
Affinity DataIC50: 3nMAssay Description:Inhibition of recombinant Syk (unknown origin)More data for this Ligand-Target Pair
Affinity DataIC50: 3.20nMAssay Description:Inhibition of recombinant AXL (unknown origin) using poly (Glu,Tyr) 4:1 as substrate incubated for 60 mins in presence of ATP by ELISAMore data for this Ligand-Target Pair
TargetMacrophage colony-stimulating factor 1 receptor(Homo sapiens (Human))
Chinese Academy Of Sciences
Curated by ChEMBL
Chinese Academy Of Sciences
Curated by ChEMBL
Affinity DataIC50: 3.40nMAssay Description:Inhibition of recombinant human His-tagged CSF1R cytoplasmic domain (538 to 910 residues) expressed in baculovirus using tyr4 peptide as substrate af...More data for this Ligand-Target Pair
Affinity DataIC50: 3.70nMAssay Description:Inhibition of recombinant Syk (unknown origin)More data for this Ligand-Target Pair
Affinity DataIC50: 4nMAssay Description:Inhibition of recombinant Syk (unknown origin)More data for this Ligand-Target Pair
Affinity DataIC50: 4nMAssay Description:Inhibition of recombinant Syk (unknown origin)More data for this Ligand-Target Pair
Affinity DataIC50: 4nMAssay Description:Inhibition of human HDAC1More data for this Ligand-Target Pair
TargetMacrophage colony-stimulating factor 1 receptor(Homo sapiens (Human))
Chinese Academy Of Sciences
Curated by ChEMBL
Chinese Academy Of Sciences
Curated by ChEMBL
Affinity DataIC50: 4.40nMAssay Description:Inhibition of recombinant human His-tagged CSF1R cytoplasmic domain (538 to 910 residues) expressed in baculovirus using tyr4 peptide as substrate af...More data for this Ligand-Target Pair
TargetMacrophage colony-stimulating factor 1 receptor(Homo sapiens (Human))
Chinese Academy Of Sciences
Curated by ChEMBL
Chinese Academy Of Sciences
Curated by ChEMBL
Affinity DataIC50: 4.5nMAssay Description:Inhibition of recombinant human His-tagged CSF1R cytoplasmic domain (538 to 910 residues) expressed in baculovirus using tyr4 peptide as substrate af...More data for this Ligand-Target Pair
TargetMacrophage colony-stimulating factor 1 receptor(Homo sapiens (Human))
Chinese Academy Of Sciences
Curated by ChEMBL
Chinese Academy Of Sciences
Curated by ChEMBL
Affinity DataIC50: 4.5nMAssay Description:Inhibition of recombinant human His-tagged CSF1R cytoplasmic domain (538 to 910 residues) expressed in baculovirus using tyr4 peptide as substrate af...More data for this Ligand-Target Pair
Affinity DataIC50: 4.5nMAssay Description:Inhibition of human HDAC1More data for this Ligand-Target Pair
TargetMacrophage colony-stimulating factor 1 receptor(Homo sapiens (Human))
Chinese Academy Of Sciences
Curated by ChEMBL
Chinese Academy Of Sciences
Curated by ChEMBL
Affinity DataIC50: 4.70nMAssay Description:Inhibition of recombinant human His-tagged CSF1R cytoplasmic domain (538 to 910 residues) expressed in baculovirus using tyr4 peptide as substrate af...More data for this Ligand-Target Pair
TargetMacrophage colony-stimulating factor 1 receptor(Homo sapiens (Human))
Chinese Academy Of Sciences
Curated by ChEMBL
Chinese Academy Of Sciences
Curated by ChEMBL
Affinity DataIC50: 4.90nMAssay Description:Inhibition of recombinant human His-tagged CSF1R cytoplasmic domain (538 to 910 residues) expressed in baculovirus using tyr4 peptide as substrate af...More data for this Ligand-Target Pair
TargetG-protein coupled receptor 183(Homo sapiens (Human))
Sanford Burnham Prebys Medical Discovery Institute
US Patent
Sanford Burnham Prebys Medical Discovery Institute
US Patent
Affinity DataIC50: <5nMAssay Description:The test compounds that demonstrated a corrected % activity of >=50% were defined as inhibitors of the reaction. The experimental values were normali...More data for this Ligand-Target Pair
TargetG-protein coupled receptor 183(Homo sapiens (Human))
Sanford Burnham Prebys Medical Discovery Institute
US Patent
Sanford Burnham Prebys Medical Discovery Institute
US Patent
Affinity DataIC50: <5nMAssay Description:The test compounds that demonstrated a corrected % activity of >=50% were defined as inhibitors of the reaction. The experimental values were normali...More data for this Ligand-Target Pair
TargetG-protein coupled receptor 183(Homo sapiens (Human))
Sanford Burnham Prebys Medical Discovery Institute
US Patent
Sanford Burnham Prebys Medical Discovery Institute
US Patent
Affinity DataIC50: <5nMAssay Description:The test compounds that demonstrated a corrected % activity of >=50% were defined as inhibitors of the reaction. The experimental values were normali...More data for this Ligand-Target Pair
TargetG-protein coupled receptor 183(Homo sapiens (Human))
Sanford Burnham Prebys Medical Discovery Institute
US Patent
Sanford Burnham Prebys Medical Discovery Institute
US Patent
Affinity DataIC50: <5nMAssay Description:The test compounds that demonstrated a corrected % activity of >=50% were defined as inhibitors of the reaction. The experimental values were normali...More data for this Ligand-Target Pair
TargetG-protein coupled receptor 183(Homo sapiens (Human))
Sanford Burnham Prebys Medical Discovery Institute
US Patent
Sanford Burnham Prebys Medical Discovery Institute
US Patent
Affinity DataIC50: <5nMAssay Description:The test compounds that demonstrated a corrected % activity of >=50% were defined as inhibitors of the reaction. The experimental values were normali...More data for this Ligand-Target Pair
TargetG-protein coupled receptor 183(Homo sapiens (Human))
Sanford Burnham Prebys Medical Discovery Institute
US Patent
Sanford Burnham Prebys Medical Discovery Institute
US Patent
Affinity DataIC50: <5nMAssay Description:The test compounds that demonstrated a corrected % activity of >=50% were defined as inhibitors of the reaction. The experimental values were normali...More data for this Ligand-Target Pair

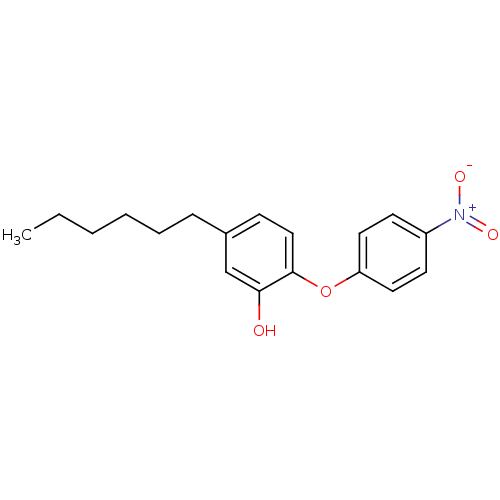

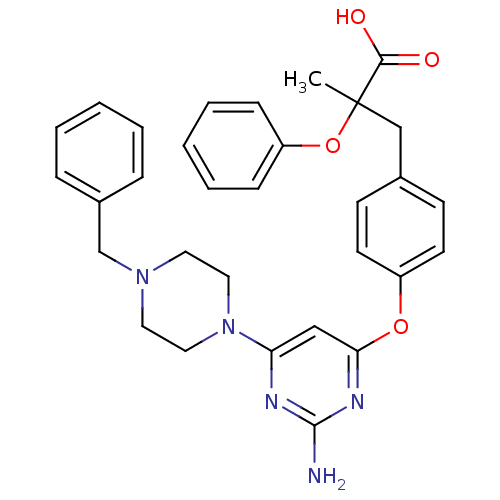
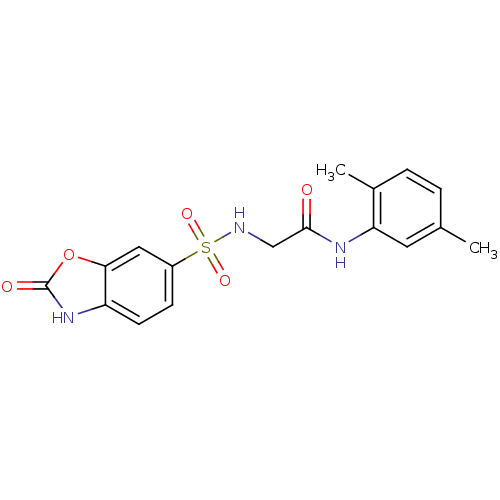
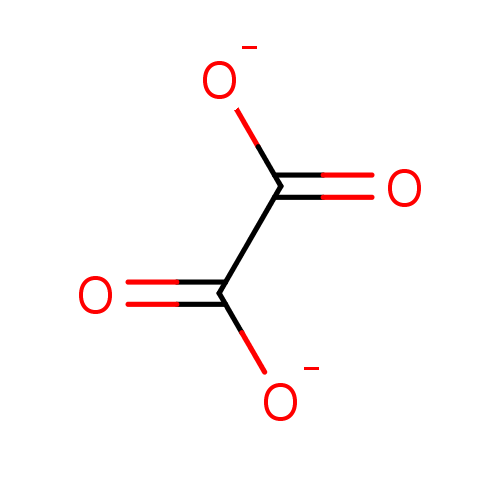
 3D Structure (crystal)
3D Structure (crystal)