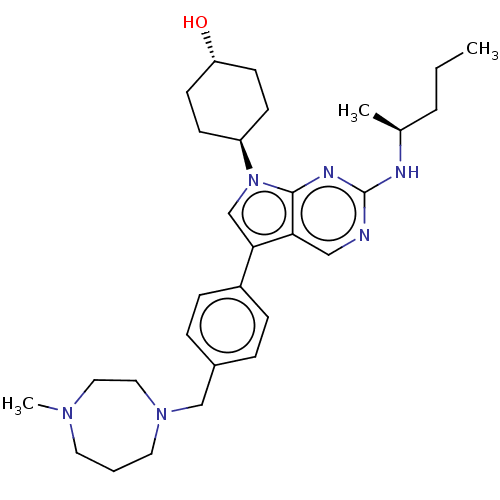
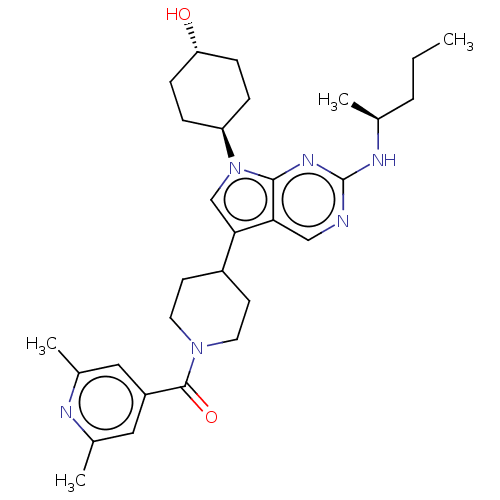
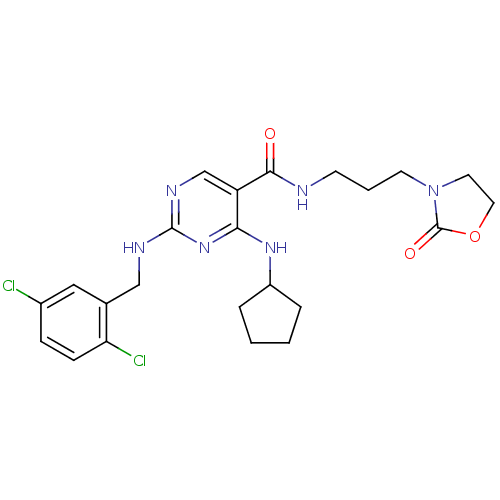
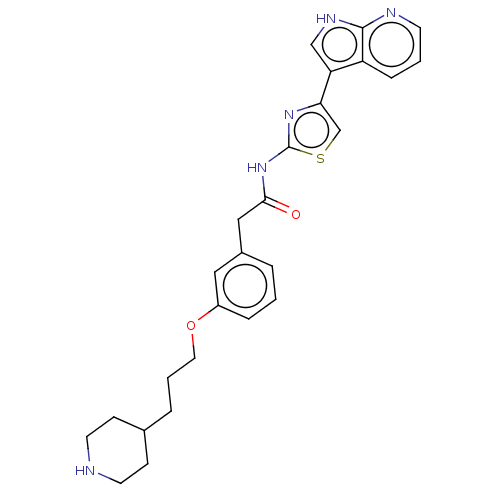
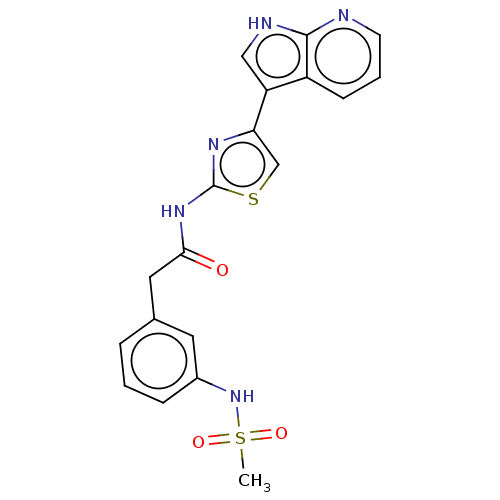
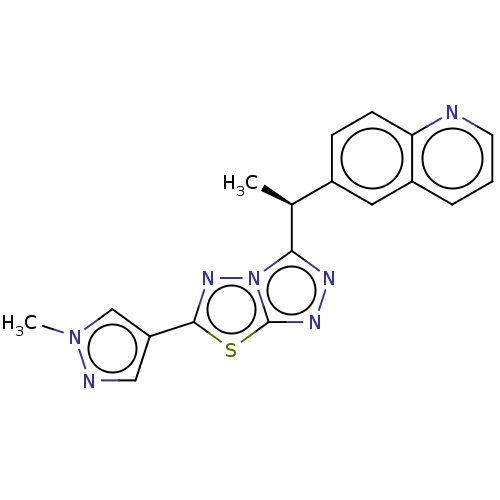
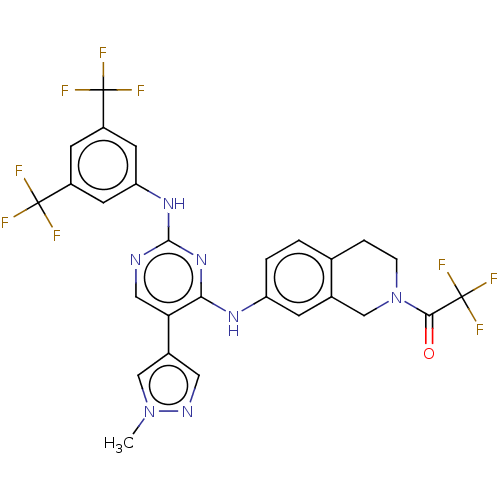
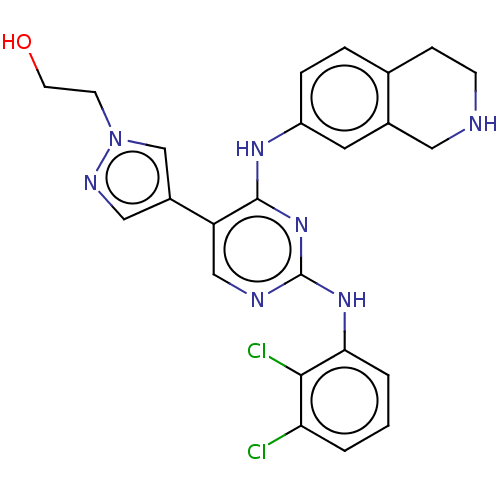
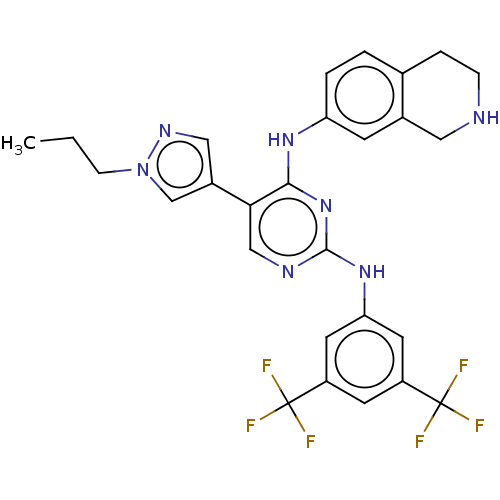
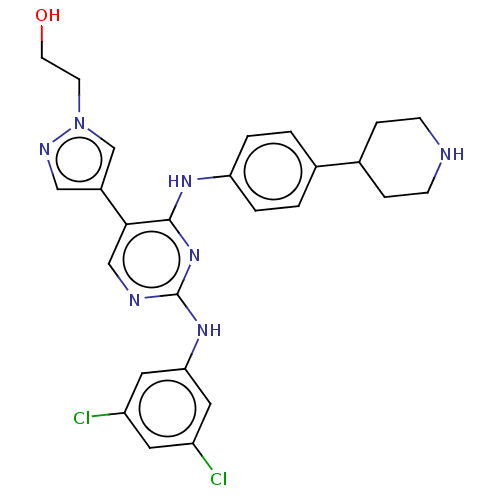
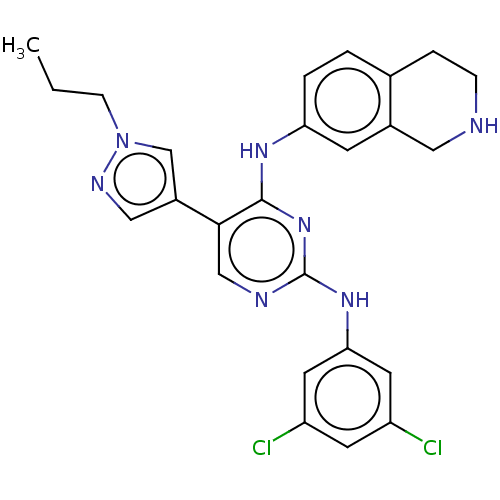
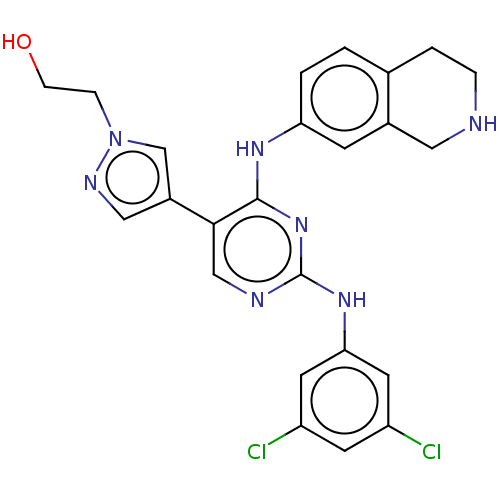
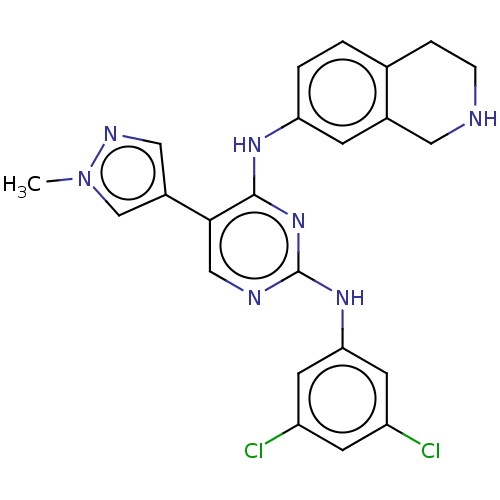
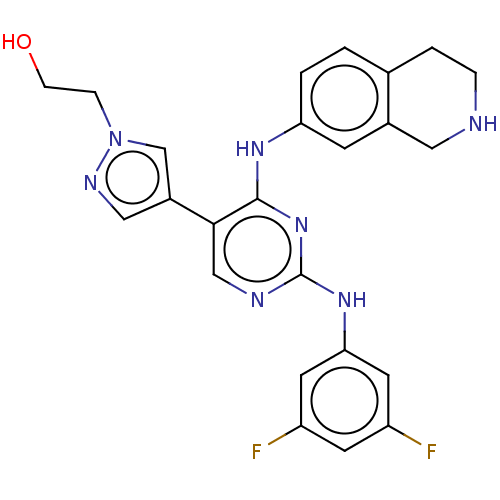
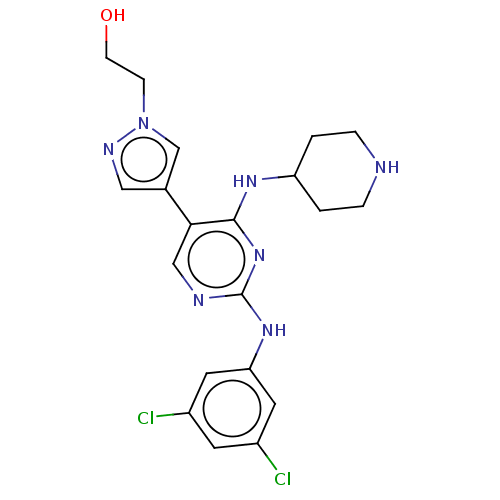
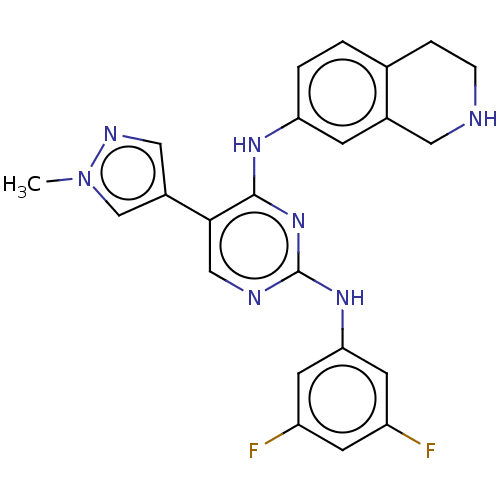
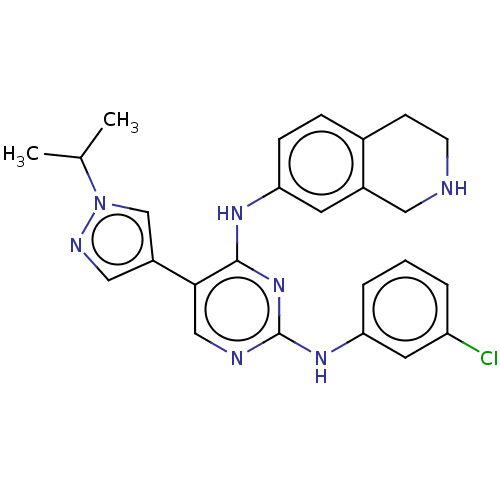
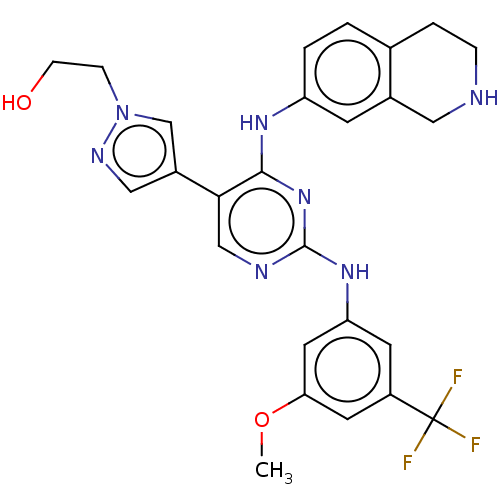
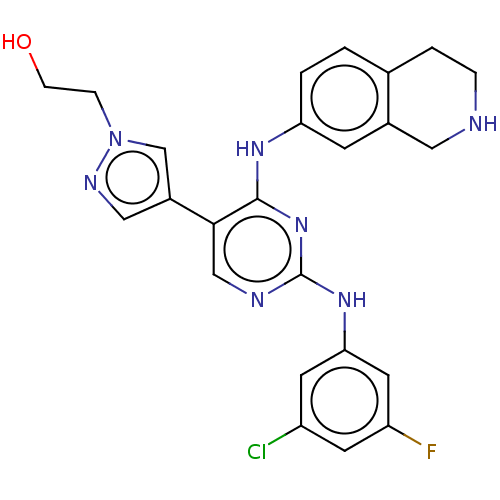
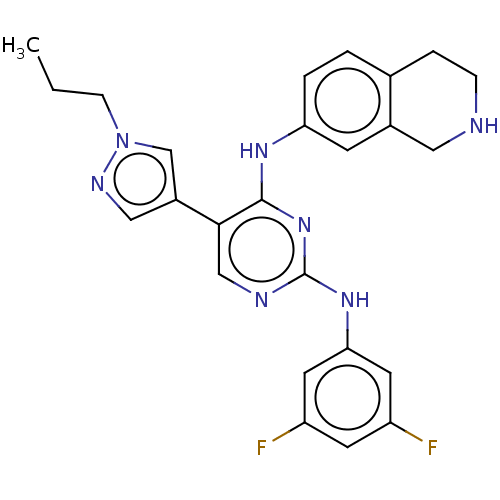
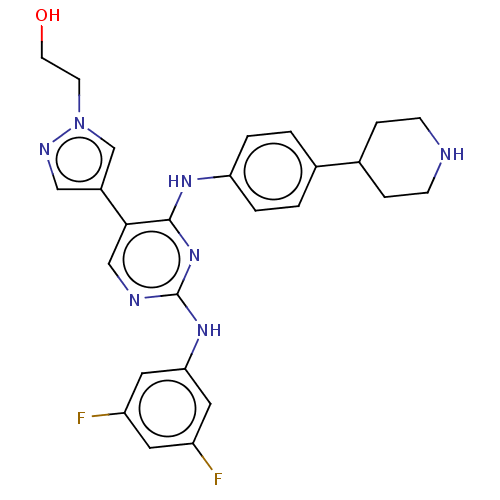
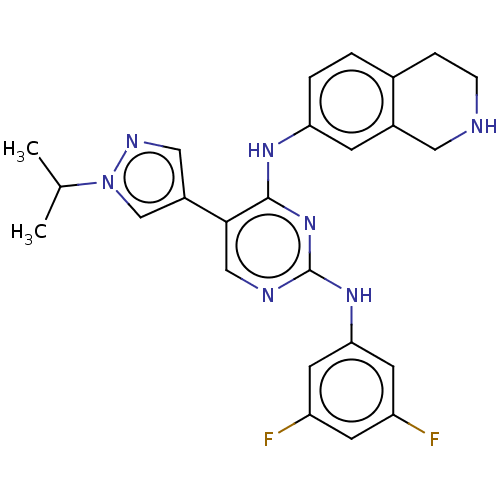
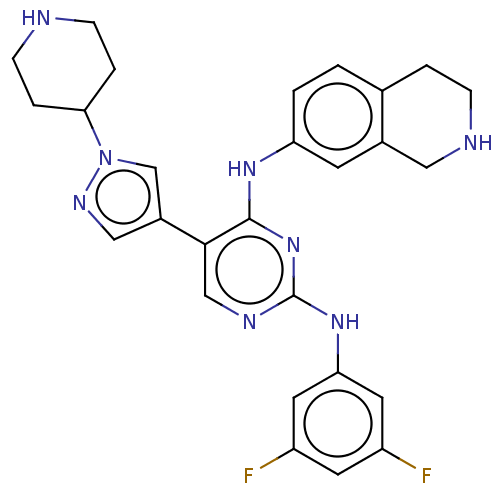
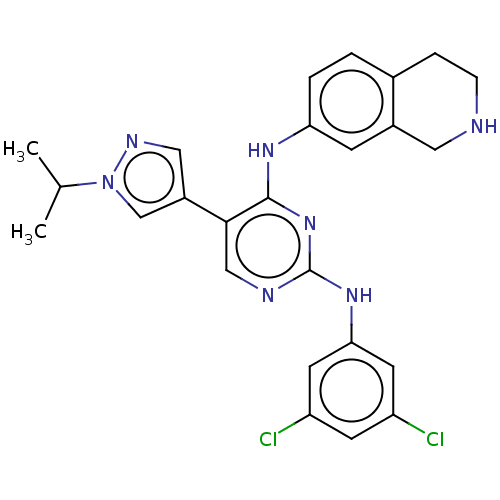
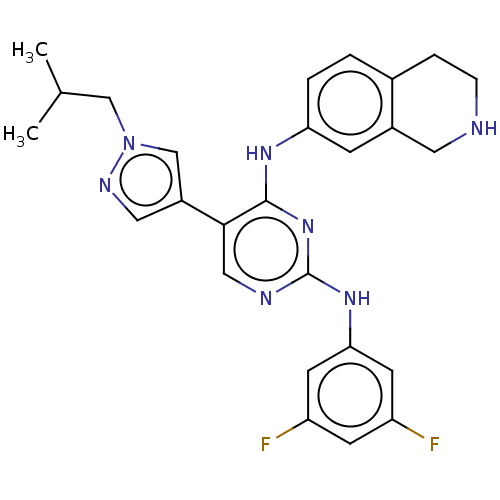
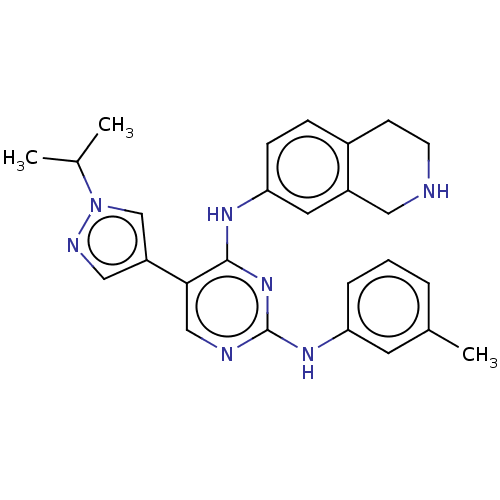
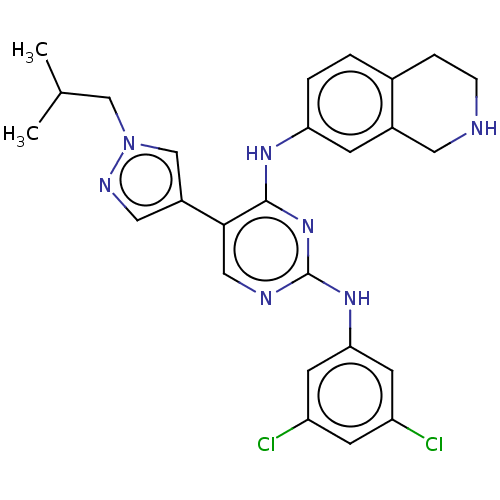
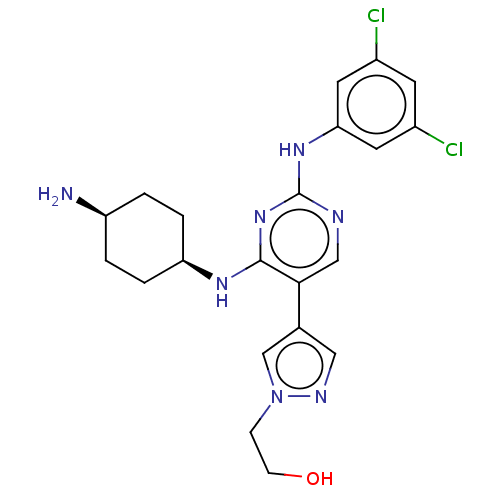
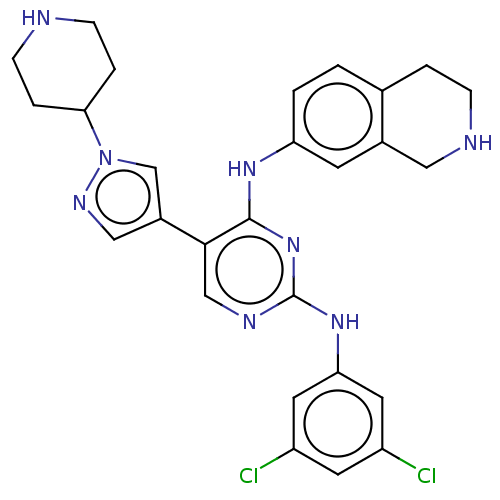
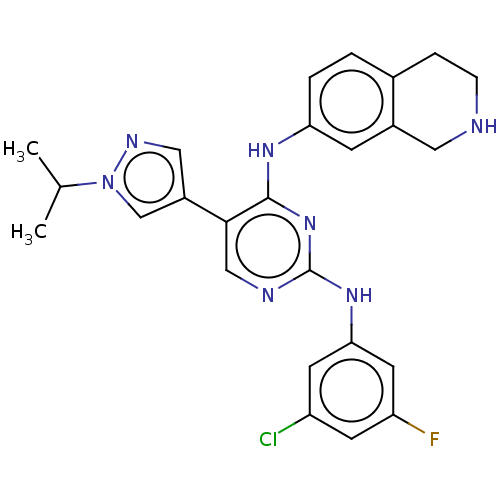
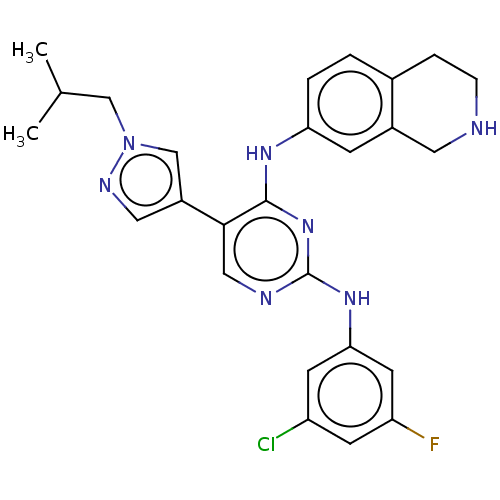
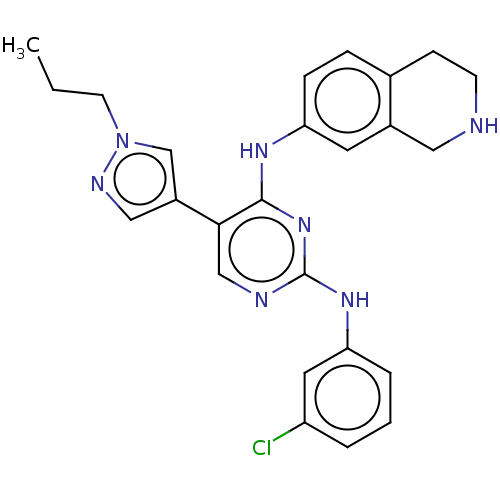
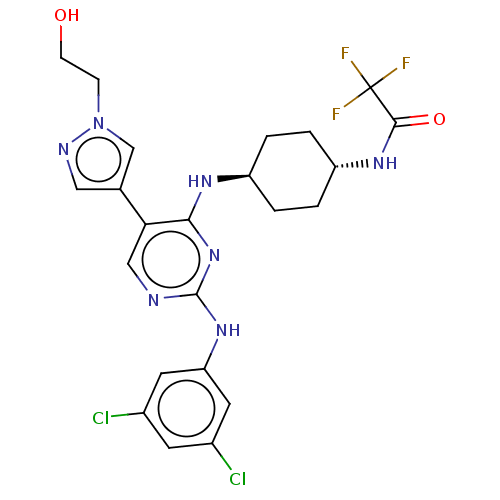
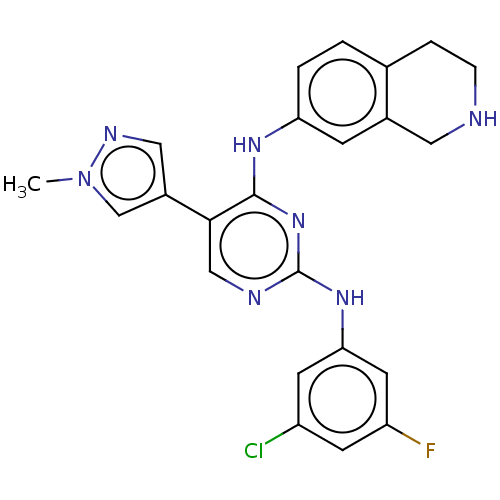
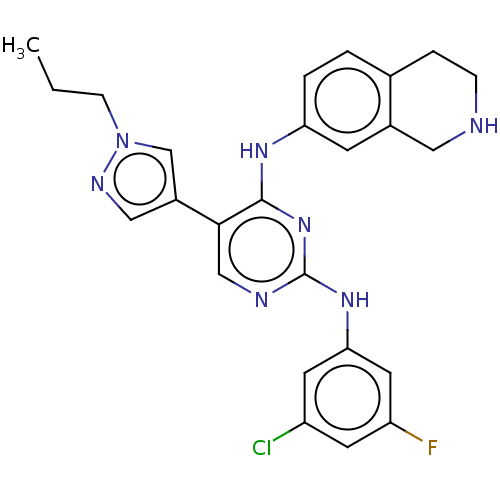
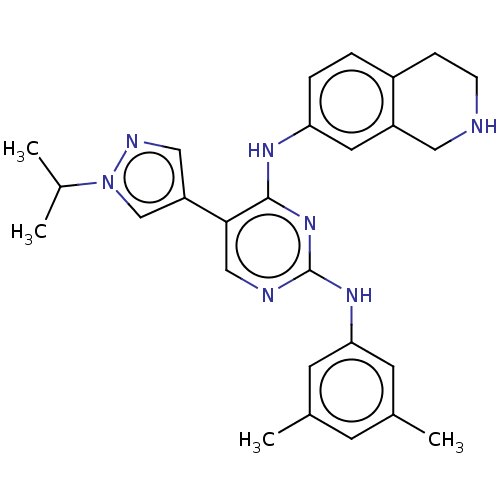
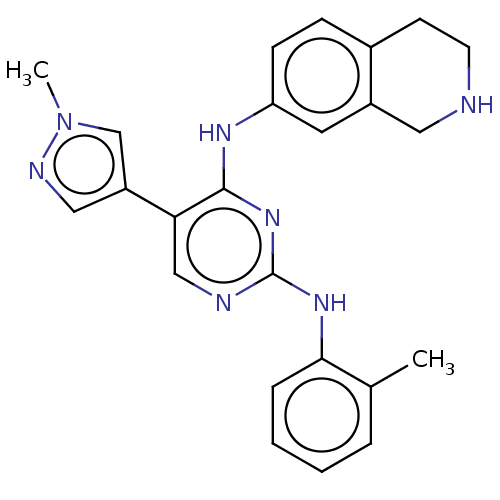
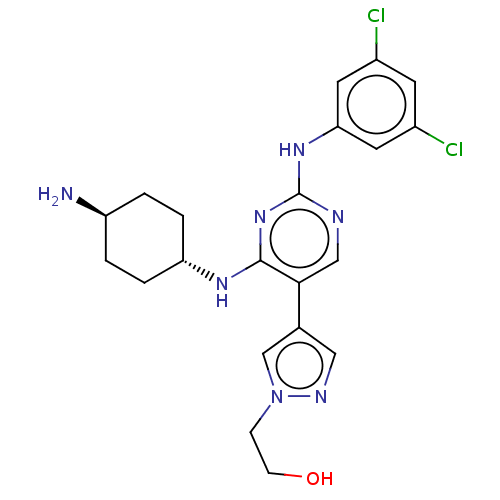
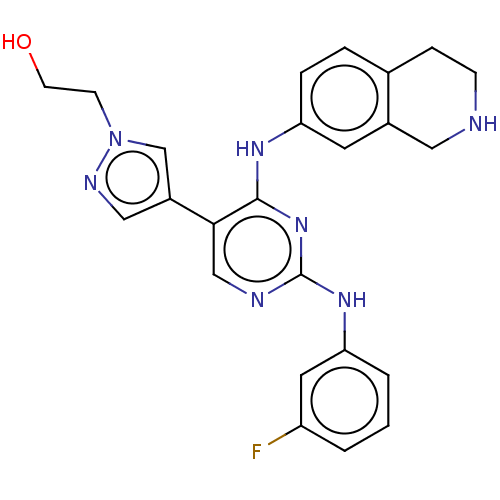
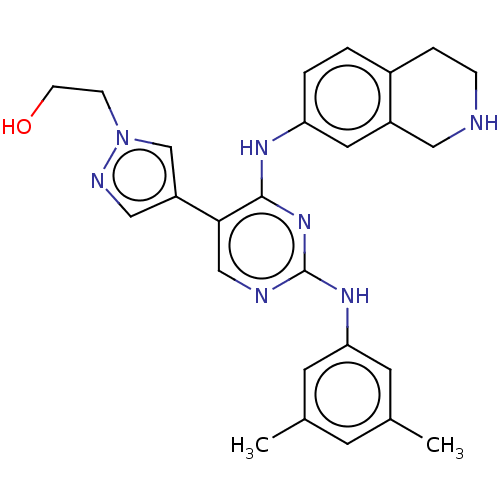
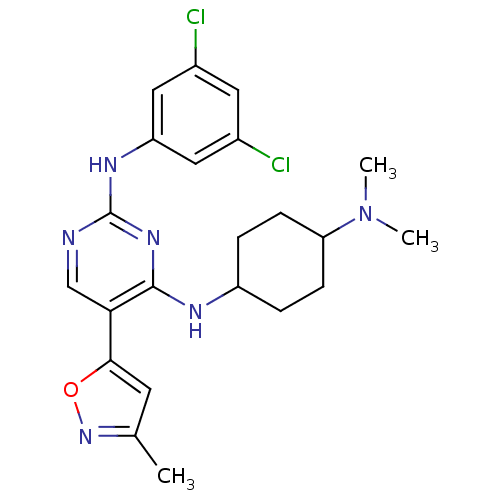
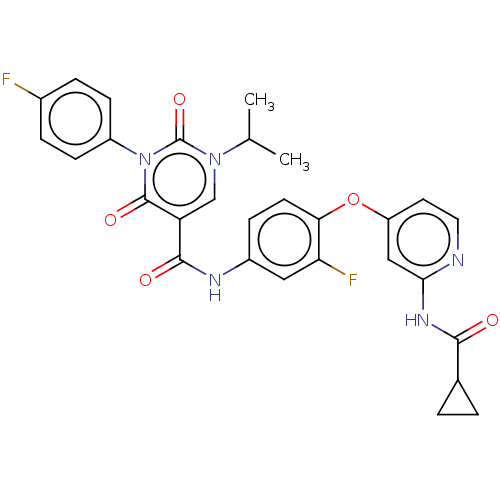
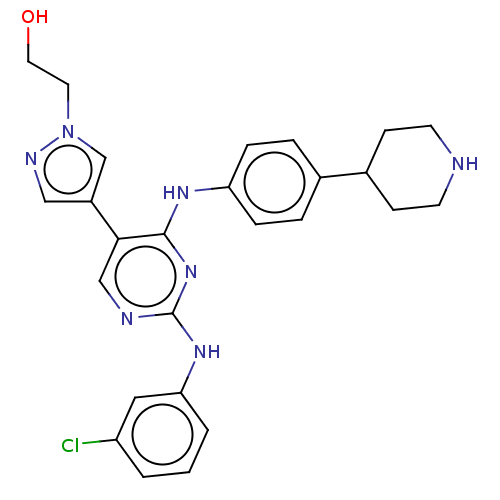
TargetTyrosine-protein kinase receptor TYRO3(Homo sapiens (Human))
Unc Eshelman School Of Pharmacy
Curated by ChEMBL
Unc Eshelman School Of Pharmacy
Curated by ChEMBL
Affinity DataKi: 3.90nMAssay Description:Inhibition of TYRO3 (unknown origin) using 5'-FAM-EFPIYDFLPAKKK-CONH2 as substrate after 180 mins by MCE assayMore data for this Ligand-Target Pair
TargetTyrosine-protein kinase receptor TYRO3(Homo sapiens (Human))
Unc Eshelman School Of Pharmacy
Curated by ChEMBL
Unc Eshelman School Of Pharmacy
Curated by ChEMBL
Affinity DataKi: 19nMAssay Description:ATP competitive inhibition of TYRO3 (unknown origin)More data for this Ligand-Target Pair
TargetTyrosine-protein kinase receptor TYRO3(Homo sapiens (Human))
Unc Eshelman School Of Pharmacy
Curated by ChEMBL
Unc Eshelman School Of Pharmacy
Curated by ChEMBL
Affinity DataKi: 120nMAssay Description:Inhibition of tracer K5 binding to NanoLuc-fused TYRO3 (unknown origin) expressed in HEK293T cells measured after 2 hrs by NanoBRET assayMore data for this Ligand-Target Pair
TargetTyrosine-protein kinase receptor TYRO3(Homo sapiens (Human))
Unc Eshelman School Of Pharmacy
Curated by ChEMBL
Unc Eshelman School Of Pharmacy
Curated by ChEMBL
Affinity DataKi: >2.00E+3nMAssay Description:Inhibition of TYRO3 (unknown origin)More data for this Ligand-Target Pair
TargetTyrosine-protein kinase receptor TYRO3(Homo sapiens (Human))
Unc Eshelman School Of Pharmacy
Curated by ChEMBL
Unc Eshelman School Of Pharmacy
Curated by ChEMBL
Affinity DataKi: >2.00E+3nMAssay Description:Inhibition of TYRO3 (unknown origin)More data for this Ligand-Target Pair
TargetTyrosine-protein kinase receptor TYRO3(Homo sapiens (Human))
Unc Eshelman School Of Pharmacy
Curated by ChEMBL
Unc Eshelman School Of Pharmacy
Curated by ChEMBL
Affinity DataKi: >4.00E+3nMAssay Description:Inhibition of SKY (unknown origin)More data for this Ligand-Target Pair
TargetTyrosine-protein kinase receptor TYRO3(Homo sapiens (Human))
Unc Eshelman School Of Pharmacy
Curated by ChEMBL
Unc Eshelman School Of Pharmacy
Curated by ChEMBL
Affinity DataIC50: 0.00600nMAssay Description:For evaluation of inhibitory activities of the compounds according to the present invention on Tyro 3, Axl, and Mer, the following test was carried o...More data for this Ligand-Target Pair
TargetTyrosine-protein kinase receptor TYRO3(Homo sapiens (Human))
Unc Eshelman School Of Pharmacy
Curated by ChEMBL
Unc Eshelman School Of Pharmacy
Curated by ChEMBL
Affinity DataIC50: 0.0100nMAssay Description:For evaluation of inhibitory activities of the compounds according to the present invention on Tyro 3, Axl, and Mer, the following test was carried o...More data for this Ligand-Target Pair
TargetTyrosine-protein kinase receptor TYRO3(Homo sapiens (Human))
Unc Eshelman School Of Pharmacy
Curated by ChEMBL
Unc Eshelman School Of Pharmacy
Curated by ChEMBL
Affinity DataIC50: 0.0120nMAssay Description:For evaluation of inhibitory activities of the compounds according to the present invention on Tyro 3, Axl, and Mer, the following test was carried o...More data for this Ligand-Target Pair
TargetTyrosine-protein kinase receptor TYRO3(Homo sapiens (Human))
Unc Eshelman School Of Pharmacy
Curated by ChEMBL
Unc Eshelman School Of Pharmacy
Curated by ChEMBL
Affinity DataIC50: 0.0120nMAssay Description:For evaluation of inhibitory activities of the compounds according to the present invention on Tyro 3, Axl, and Mer, the following test was carried o...More data for this Ligand-Target Pair
TargetTyrosine-protein kinase receptor TYRO3(Homo sapiens (Human))
Unc Eshelman School Of Pharmacy
Curated by ChEMBL
Unc Eshelman School Of Pharmacy
Curated by ChEMBL
Affinity DataIC50: 0.0120nMAssay Description:For evaluation of inhibitory activities of the compounds according to the present invention on Tyro 3, Axl, and Mer, the following test was carried o...More data for this Ligand-Target Pair
TargetTyrosine-protein kinase receptor TYRO3(Homo sapiens (Human))
Unc Eshelman School Of Pharmacy
Curated by ChEMBL
Unc Eshelman School Of Pharmacy
Curated by ChEMBL
Affinity DataIC50: 0.0120nMAssay Description:For evaluation of inhibitory activities of the compounds according to the present invention on Tyro 3, Axl, and Mer, the following test was carried o...More data for this Ligand-Target Pair
TargetTyrosine-protein kinase receptor TYRO3(Homo sapiens (Human))
Unc Eshelman School Of Pharmacy
Curated by ChEMBL
Unc Eshelman School Of Pharmacy
Curated by ChEMBL
Affinity DataIC50: 0.0410nMAssay Description:For evaluation of inhibitory activities of the compounds according to the present invention on Tyro 3, Axl, and Mer, the following test was carried o...More data for this Ligand-Target Pair
TargetTyrosine-protein kinase receptor TYRO3(Homo sapiens (Human))
Unc Eshelman School Of Pharmacy
Curated by ChEMBL
Unc Eshelman School Of Pharmacy
Curated by ChEMBL
Affinity DataIC50: 0.0410nMAssay Description:For evaluation of inhibitory activities of the compounds according to the present invention on Tyro 3, Axl, and Mer, the following test was carried o...More data for this Ligand-Target Pair
TargetTyrosine-protein kinase receptor TYRO3(Homo sapiens (Human))
Unc Eshelman School Of Pharmacy
Curated by ChEMBL
Unc Eshelman School Of Pharmacy
Curated by ChEMBL
Affinity DataIC50: 0.0520nMAssay Description:For evaluation of inhibitory activities of the compounds according to the present invention on Tyro 3, Axl, and Mer, the following test was carried o...More data for this Ligand-Target Pair
TargetTyrosine-protein kinase receptor TYRO3(Homo sapiens (Human))
Unc Eshelman School Of Pharmacy
Curated by ChEMBL
Unc Eshelman School Of Pharmacy
Curated by ChEMBL
Affinity DataIC50: 0.0600nMAssay Description:For evaluation of inhibitory activities of the compounds according to the present invention on Tyro 3, Axl, and Mer, the following test was carried o...More data for this Ligand-Target Pair
TargetTyrosine-protein kinase receptor TYRO3(Homo sapiens (Human))
Unc Eshelman School Of Pharmacy
Curated by ChEMBL
Unc Eshelman School Of Pharmacy
Curated by ChEMBL
Affinity DataIC50: 0.0970nMAssay Description:For evaluation of inhibitory activities of the compounds according to the present invention on Tyro 3, Axl, and Mer, the following test was carried o...More data for this Ligand-Target Pair
TargetTyrosine-protein kinase receptor TYRO3(Homo sapiens (Human))
Unc Eshelman School Of Pharmacy
Curated by ChEMBL
Unc Eshelman School Of Pharmacy
Curated by ChEMBL
Affinity DataIC50: 0.100nMAssay Description:For evaluation of inhibitory activities of the compounds according to the present invention on Tyro 3, Axl, and Mer, the following test was carried o...More data for this Ligand-Target Pair
TargetTyrosine-protein kinase receptor TYRO3(Homo sapiens (Human))
Unc Eshelman School Of Pharmacy
Curated by ChEMBL
Unc Eshelman School Of Pharmacy
Curated by ChEMBL
Affinity DataIC50: 0.130nMAssay Description:For evaluation of inhibitory activities of the compounds according to the present invention on Tyro 3, Axl, and Mer, the following test was carried o...More data for this Ligand-Target Pair
TargetTyrosine-protein kinase receptor TYRO3(Homo sapiens (Human))
Unc Eshelman School Of Pharmacy
Curated by ChEMBL
Unc Eshelman School Of Pharmacy
Curated by ChEMBL
Affinity DataIC50: 0.130nMAssay Description:For evaluation of inhibitory activities of the compounds according to the present invention on Tyro 3, Axl, and Mer, the following test was carried o...More data for this Ligand-Target Pair
TargetTyrosine-protein kinase receptor TYRO3(Homo sapiens (Human))
Unc Eshelman School Of Pharmacy
Curated by ChEMBL
Unc Eshelman School Of Pharmacy
Curated by ChEMBL
Affinity DataIC50: 0.130nMAssay Description:For evaluation of inhibitory activities of the compounds according to the present invention on Tyro 3, Axl, and Mer, the following test was carried o...More data for this Ligand-Target Pair
TargetTyrosine-protein kinase receptor TYRO3(Homo sapiens (Human))
Unc Eshelman School Of Pharmacy
Curated by ChEMBL
Unc Eshelman School Of Pharmacy
Curated by ChEMBL
Affinity DataIC50: 0.140nMAssay Description:For evaluation of inhibitory activities of the compounds according to the present invention on Tyro 3, Axl, and Mer, the following test was carried o...More data for this Ligand-Target Pair
TargetTyrosine-protein kinase receptor TYRO3(Homo sapiens (Human))
Unc Eshelman School Of Pharmacy
Curated by ChEMBL
Unc Eshelman School Of Pharmacy
Curated by ChEMBL
Affinity DataIC50: 0.140nMAssay Description:For evaluation of inhibitory activities of the compounds according to the present invention on Tyro 3, Axl, and Mer, the following test was carried o...More data for this Ligand-Target Pair
TargetTyrosine-protein kinase receptor TYRO3(Homo sapiens (Human))
Unc Eshelman School Of Pharmacy
Curated by ChEMBL
Unc Eshelman School Of Pharmacy
Curated by ChEMBL
Affinity DataIC50: 0.160nMAssay Description:For evaluation of inhibitory activities of the compounds according to the present invention on Tyro 3, Axl, and Mer, the following test was carried o...More data for this Ligand-Target Pair
TargetTyrosine-protein kinase receptor TYRO3(Homo sapiens (Human))
Unc Eshelman School Of Pharmacy
Curated by ChEMBL
Unc Eshelman School Of Pharmacy
Curated by ChEMBL
Affinity DataIC50: 0.160nMAssay Description:For evaluation of inhibitory activities of the compounds according to the present invention on Tyro 3, Axl, and Mer, the following test was carried o...More data for this Ligand-Target Pair
TargetTyrosine-protein kinase receptor TYRO3(Homo sapiens (Human))
Unc Eshelman School Of Pharmacy
Curated by ChEMBL
Unc Eshelman School Of Pharmacy
Curated by ChEMBL
Affinity DataIC50: 0.160nMAssay Description:For evaluation of inhibitory activities of the compounds according to the present invention on Tyro 3, Axl, and Mer, the following test was carried o...More data for this Ligand-Target Pair
TargetTyrosine-protein kinase receptor TYRO3(Homo sapiens (Human))
Unc Eshelman School Of Pharmacy
Curated by ChEMBL
Unc Eshelman School Of Pharmacy
Curated by ChEMBL
Affinity DataIC50: 0.170nMAssay Description:For evaluation of inhibitory activities of the compounds according to the present invention on Tyro 3, Axl, and Mer, the following test was carried o...More data for this Ligand-Target Pair
TargetTyrosine-protein kinase receptor TYRO3(Homo sapiens (Human))
Unc Eshelman School Of Pharmacy
Curated by ChEMBL
Unc Eshelman School Of Pharmacy
Curated by ChEMBL
Affinity DataIC50: 0.170nMAssay Description:For evaluation of inhibitory activities of the compounds according to the present invention on Tyro 3, Axl, and Mer, the following test was carried o...More data for this Ligand-Target Pair
TargetTyrosine-protein kinase receptor TYRO3(Homo sapiens (Human))
Unc Eshelman School Of Pharmacy
Curated by ChEMBL
Unc Eshelman School Of Pharmacy
Curated by ChEMBL
Affinity DataIC50: 0.170nMAssay Description:For evaluation of inhibitory activities of the compounds according to the present invention on Tyro 3, Axl, and Mer, the following test was carried o...More data for this Ligand-Target Pair
TargetTyrosine-protein kinase receptor TYRO3(Homo sapiens (Human))
Unc Eshelman School Of Pharmacy
Curated by ChEMBL
Unc Eshelman School Of Pharmacy
Curated by ChEMBL
Affinity DataIC50: 0.190nMAssay Description:For evaluation of inhibitory activities of the compounds according to the present invention on Tyro 3, Axl, and Mer, the following test was carried o...More data for this Ligand-Target Pair
TargetTyrosine-protein kinase receptor TYRO3(Homo sapiens (Human))
Unc Eshelman School Of Pharmacy
Curated by ChEMBL
Unc Eshelman School Of Pharmacy
Curated by ChEMBL
Affinity DataIC50: 0.200nMAssay Description:For evaluation of inhibitory activities of the compounds according to the present invention on Tyro 3, Axl, and Mer, the following test was carried o...More data for this Ligand-Target Pair
TargetTyrosine-protein kinase receptor TYRO3(Homo sapiens (Human))
Unc Eshelman School Of Pharmacy
Curated by ChEMBL
Unc Eshelman School Of Pharmacy
Curated by ChEMBL
Affinity DataIC50: 0.230nMAssay Description:For evaluation of inhibitory activities of the compounds according to the present invention on Tyro 3, Axl, and Mer, the following test was carried o...More data for this Ligand-Target Pair
TargetTyrosine-protein kinase receptor TYRO3(Homo sapiens (Human))
Unc Eshelman School Of Pharmacy
Curated by ChEMBL
Unc Eshelman School Of Pharmacy
Curated by ChEMBL
Affinity DataIC50: 0.270nMAssay Description:For evaluation of inhibitory activities of the compounds according to the present invention on Tyro 3, Axl, and Mer, the following test was carried o...More data for this Ligand-Target Pair
TargetTyrosine-protein kinase receptor TYRO3(Homo sapiens (Human))
Unc Eshelman School Of Pharmacy
Curated by ChEMBL
Unc Eshelman School Of Pharmacy
Curated by ChEMBL
Affinity DataIC50: 0.270nMAssay Description:For evaluation of inhibitory activities of the compounds according to the present invention on Tyro 3, Axl, and Mer, the following test was carried o...More data for this Ligand-Target Pair
TargetTyrosine-protein kinase receptor TYRO3(Homo sapiens (Human))
Unc Eshelman School Of Pharmacy
Curated by ChEMBL
Unc Eshelman School Of Pharmacy
Curated by ChEMBL
Affinity DataIC50: 0.280nMAssay Description:For evaluation of inhibitory activities of the compounds according to the present invention on Tyro 3, Axl, and Mer, the following test was carried o...More data for this Ligand-Target Pair
TargetTyrosine-protein kinase receptor TYRO3(Homo sapiens (Human))
Unc Eshelman School Of Pharmacy
Curated by ChEMBL
Unc Eshelman School Of Pharmacy
Curated by ChEMBL
Affinity DataIC50: 0.300nMAssay Description:For evaluation of inhibitory activities of the compounds according to the present invention on Tyro 3, Axl, and Mer, the following test was carried o...More data for this Ligand-Target Pair
TargetTyrosine-protein kinase receptor TYRO3(Homo sapiens (Human))
Unc Eshelman School Of Pharmacy
Curated by ChEMBL
Unc Eshelman School Of Pharmacy
Curated by ChEMBL
Affinity DataIC50: 0.320nMAssay Description:For evaluation of inhibitory activities of the compounds according to the present invention on Tyro 3, Axl, and Mer, the following test was carried o...More data for this Ligand-Target Pair
TargetTyrosine-protein kinase receptor TYRO3(Homo sapiens (Human))
Unc Eshelman School Of Pharmacy
Curated by ChEMBL
Unc Eshelman School Of Pharmacy
Curated by ChEMBL
Affinity DataIC50: 0.340nMAssay Description:For evaluation of inhibitory activities of the compounds according to the present invention on Tyro 3, Axl, and Mer, the following test was carried o...More data for this Ligand-Target Pair
TargetTyrosine-protein kinase receptor TYRO3(Homo sapiens (Human))
Unc Eshelman School Of Pharmacy
Curated by ChEMBL
Unc Eshelman School Of Pharmacy
Curated by ChEMBL
Affinity DataIC50: 0.340nMAssay Description:For evaluation of inhibitory activities of the compounds according to the present invention on Tyro 3, Axl, and Mer, the following test was carried o...More data for this Ligand-Target Pair
TargetTyrosine-protein kinase receptor TYRO3(Homo sapiens (Human))
Unc Eshelman School Of Pharmacy
Curated by ChEMBL
Unc Eshelman School Of Pharmacy
Curated by ChEMBL
Affinity DataIC50: 0.370nMAssay Description:For evaluation of inhibitory activities of the compounds according to the present invention on Tyro 3, Axl, and Mer, the following test was carried o...More data for this Ligand-Target Pair
TargetTyrosine-protein kinase receptor TYRO3(Homo sapiens (Human))
Unc Eshelman School Of Pharmacy
Curated by ChEMBL
Unc Eshelman School Of Pharmacy
Curated by ChEMBL
Affinity DataIC50: 0.420nMAssay Description:For evaluation of inhibitory activities of the compounds according to the present invention on Tyro 3, Axl, and Mer, the following test was carried o...More data for this Ligand-Target Pair
TargetTyrosine-protein kinase receptor TYRO3(Homo sapiens (Human))
Unc Eshelman School Of Pharmacy
Curated by ChEMBL
Unc Eshelman School Of Pharmacy
Curated by ChEMBL
Affinity DataIC50: 0.480nMAssay Description:For evaluation of inhibitory activities of the compounds according to the present invention on Tyro 3, Axl, and Mer, the following test was carried o...More data for this Ligand-Target Pair
TargetTyrosine-protein kinase receptor TYRO3(Homo sapiens (Human))
Unc Eshelman School Of Pharmacy
Curated by ChEMBL
Unc Eshelman School Of Pharmacy
Curated by ChEMBL
Affinity DataIC50: 0.580nMAssay Description:For evaluation of inhibitory activities of the compounds according to the present invention on Tyro 3, Axl, and Mer, the following test was carried o...More data for this Ligand-Target Pair
TargetTyrosine-protein kinase receptor TYRO3(Homo sapiens (Human))
Unc Eshelman School Of Pharmacy
Curated by ChEMBL
Unc Eshelman School Of Pharmacy
Curated by ChEMBL
Affinity DataIC50: 0.600nMAssay Description:For evaluation of inhibitory activities of the compounds according to the present invention on Tyro 3, Axl, and Mer, the following test was carried o...More data for this Ligand-Target Pair
TargetTyrosine-protein kinase receptor TYRO3(Homo sapiens (Human))
Unc Eshelman School Of Pharmacy
Curated by ChEMBL
Unc Eshelman School Of Pharmacy
Curated by ChEMBL
Affinity DataIC50: 0.700nMAssay Description:Inhibition of Sky (unknown origin) by ELISA kinase assay in presence of 60 uM ATPMore data for this Ligand-Target Pair
TargetTyrosine-protein kinase receptor TYRO3(Homo sapiens (Human))
Unc Eshelman School Of Pharmacy
Curated by ChEMBL
Unc Eshelman School Of Pharmacy
Curated by ChEMBL
Affinity DataIC50: 0.900nMAssay Description:For evaluation of inhibitory activities of the compounds according to the present invention on Tyro 3, Axl, and Mer, the following test was carried o...More data for this Ligand-Target Pair
TargetTyrosine-protein kinase receptor TYRO3(Homo sapiens (Human))
Unc Eshelman School Of Pharmacy
Curated by ChEMBL
Unc Eshelman School Of Pharmacy
Curated by ChEMBL
Affinity DataIC50: 1nMAssay Description:For evaluation of inhibitory activities of the compounds according to the present invention on Tyro 3, Axl, and Mer, the following test was carried o...More data for this Ligand-Target Pair
TargetTyrosine-protein kinase receptor TYRO3(Homo sapiens (Human))
Unc Eshelman School Of Pharmacy
Curated by ChEMBL
Unc Eshelman School Of Pharmacy
Curated by ChEMBL
Affinity DataIC50: 1nMAssay Description:Inhibition of recombinant human RSE (451 to end residues) using KVEKIGEGTYGVVYK as substrate incubated for 40 mins in presence of [gamma33P]ATP by sc...More data for this Ligand-Target Pair
TargetTyrosine-protein kinase receptor TYRO3(Homo sapiens (Human))
Unc Eshelman School Of Pharmacy
Curated by ChEMBL
Unc Eshelman School Of Pharmacy
Curated by ChEMBL
Affinity DataIC50: 1.20nMAssay Description:For evaluation of inhibitory activities of the compounds according to the present invention on Tyro 3, Axl, and Mer, the following test was carried o...More data for this Ligand-Target Pair
TargetTyrosine-protein kinase receptor TYRO3(Homo sapiens (Human))
Unc Eshelman School Of Pharmacy
Curated by ChEMBL
Unc Eshelman School Of Pharmacy
Curated by ChEMBL
Affinity DataIC50: 1.30nMAssay Description:For evaluation of inhibitory activities of the compounds according to the present invention on Tyro 3, Axl, and Mer, the following test was carried o...More data for this Ligand-Target Pair