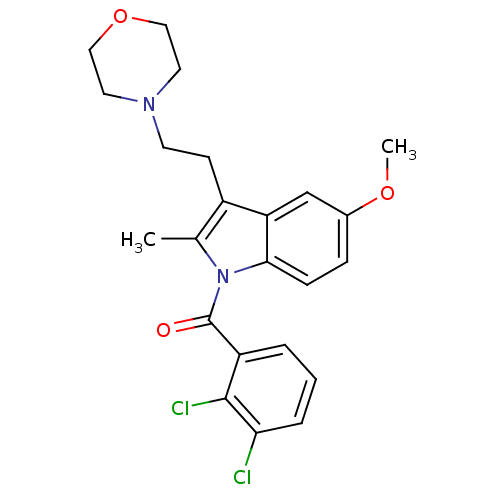
Affinity DataKi: 1.92E+3nMAssay Description:Displacement of [3H]CP 55940 from human CB1 receptor in cell free systemMore data for this Ligand-Target Pair
Affinity DataKi: 1.92E+3nMAssay Description:Binding affinity to CB1 receptorMore data for this Ligand-Target Pair
Affinity DataKi: 1.92E+3nMAssay Description:Evaluated for binding affinity against recombinant human central cannabinoid receptor 1More data for this Ligand-Target Pair
Affinity DataKi: 2.00E+3nMAssay Description:Binding affinity to human CB1 receptorMore data for this Ligand-Target Pair
Affinity DataKi: 4.80E+3nMAssay Description:Agonist activity at human CB1 receptorMore data for this Ligand-Target Pair
Affinity DataKi: 6.00E+3nMAssay Description:Binding affinity to human cannabinoid CB1 receptorMore data for this Ligand-Target Pair